Which isotope pairs were originally used to determine the age of the Earth from meteorites? Be specific and be sure to write the numbers and the elements here as listed in Table 1.
Which isotope pairs were originally used to determine the age of the Earth from meteorites? Be specific and be sure to write the numbers and the elements here as listed in Table 1.
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question

Transcribed Image Text:Which isotope pairs were originally used to determine the age of the Earth from meteorites?
Be specific and be sure to write the numbers and the elements here as listed in Table 1.
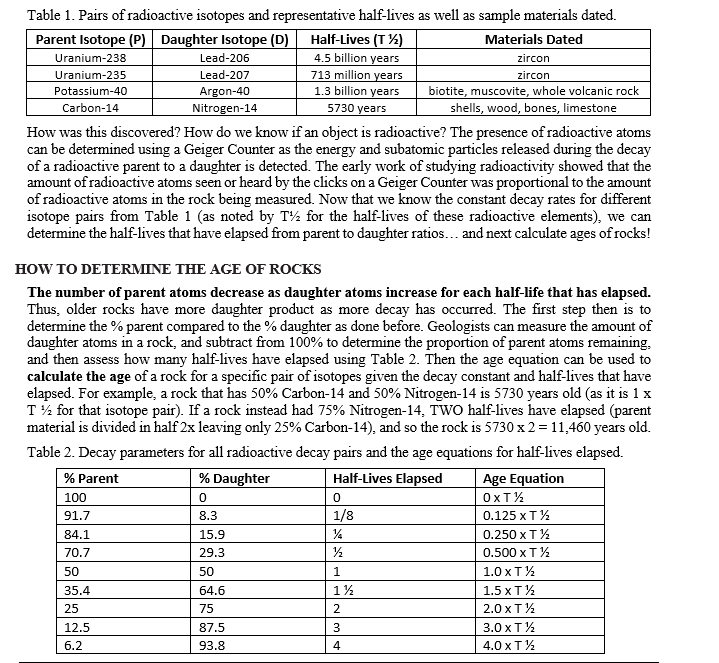
Transcribed Image Text:Table 1. Pairs of radioactive isotopes and representative half-lives as well as sample materials dated.
Half-Lives (T1½)
Materials Dated
4.5 billion years
713 million years
1.3 billion years
5730 years
Parent Isotope (P) Daughter Isotope (D)
Uranium-238
Uranium-235
Potassium-40
Carbon-14
% Parent
100
91.7
Lead-206
Lead-207
Argon-40
Nitrogen-14
How was this discovered? How do we know if an object is radioactive? The presence of radioactive atoms
can be determined using a Geiger Counter as the energy and subatomic particles released during the decay
of a radioactive parent to a daughter is detected. The early work of studying radioactivity showed that the
amount of radioactive atoms seen or heard by the clicks on a Geiger Counter was proportional to the amount
of radioactive atoms in the rock being measured. Now that we know the constant decay rates for different
isotope pairs from Table 1 (as noted by T for the half-lives of these radioactive elements), we can
determine the half-lives that have elapsed from parent to daughter ratios... and next calculate ages of rocks!
84.1
70.7
50
35.4
25
HOW TO DETERMINE THE AGE OF ROCKS
The number of parent atoms decrease as daughter atoms increase for each half-life that has elapsed.
Thus, older rocks have more daughter product as more decay has occurred. The first step then is to
determine the % parent compared to the % daughter as done before. Geologists can measure the amount of
daughter atoms in a rock, and subtract from 100% to determine the proportion of parent atoms remaining.
and then assess how many half-lives have elapsed using Table 2. Then the age equation can be used to
calculate the age of a rock for a specific pair of isotopes given the decay constant and half-lives that have
elapsed. For example, a rock that has 50% Carbon-14 and 50% Nitrogen-14 is 5730 years old (as it is 1 x
T ½ for that isotope pair). If a rock instead had 75% Nitrogen-14, TWO half-lives have elapsed (parent
material is divided in half 2x leaving only 25% Carbon-14), and so the rock is 5730 x 2 = 11,460 years old.
Table 2. Decay parameters for all radioactive decay pairs and the age equations for half-lives elapsed.
Half-Lives Elapsed
12.5
6.2
% Daughter
0
8.3
15.9
29.3
zircon
zircon
50
64.6
75
87.5
93.8
biotite, muscovite, whole volcanic rock
shells, wood, bones, limestone
0
1/8
¼
½
1
1½
2
3
4
Age Equation
0xT½
0.125 x T ½
0.250 x T ½
0.500 x T ½
1.0 x T ½
1.5 x T ½
2.0 x T ½
3.0 x T ½
4.0 x T ½
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,