Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter3: Chemical Bonds
Section: Chapter Questions
Problem 3.109P: 3-109 Until several years ago, the two chlorofluorocarbons (CFCs) most widely used as heat transfer...
Related questions
Question
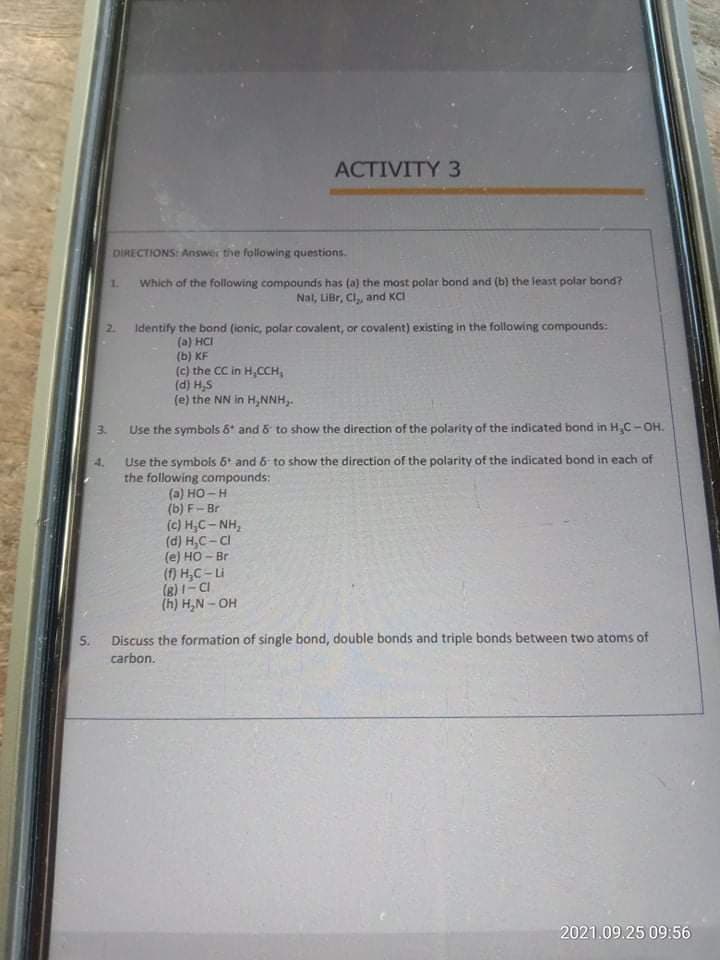
Transcribed Image Text:ACTIVITY 3
DİRECTIONS: Answer the following questions.
Which of the following compounds has (a) the most polar bond and (b) the least polar bond?
Nal, LiBr, Cl,, and KCI
1.
2.
Identify the bond (ionic, polar covalent, or covalent) existing in the following compounds:
(a) HCI
(b) KF
(c) the CC in H,CCH,
(d) H,S
(e) the NN in H,NNH,.
3.
Use the symbols 6* and & to show the direction of the polarity of the indicated bond in H,C-OH.
Use the symbois 6 and 6 to show the direction of the polarity of the indicated bond in each of
the following compounds:
(a) HO-H
(b) F- Br
(c) H,C- NH,
(d) H,C- CI
(e) HO - Br
() H,C-Li
(8)1- CI
(h) H,N - OH
S.
Discuss the formation of single bond, double bonds and triple bonds between two atoms of
carbon.
2021.09.25 09:56
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning