Which of the following compounds has the highest boiling point? hydogan 2 H Не 1009 hum 4.000 bendm bon carton rogen Oygen fne neon 5 B 3 10 Li Be N F Ne 6941 sodum 12011 slcon 14 14007 phonpone 15 15999 sultur 16 20.10 magnestm 12 al m 13 chiorine argon 11 17 18 Na Mg Al Si P S ci Ar 2430 ca 20 220 354 trome ndm 21 va m 23 pmanm 32 Mankm mongane 25 cobal 27 rkel krypn 36 polassum opper 19 22 24 26 28 29 30 31 33 34 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 40.0 4.0 am 7241 entm 44 palam 46 she cadam 48 antinony 51 utn 52 edine 53 won 37 38 39 40 41 42 43 45 47 49 50 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Хе sor 1212 casm 55 hahm 72 1124 meny 80 han 81 1260 aste 85 aon 86 bartm ngtm Iad 56 57-70 71 73 74 75 76 77 78 79 82 83 84 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 13291 anm 87 16.21 200 wtorgum 106 hassim 108 metnertum unum unuunm 110 bohrum 88 89-102 103 104 105 107 109 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uug lantham 57 centum 58 neodmtm 60 promettm 61 dypm 66 tum 68 st m 70 samanm europum gadoam 64 terbm *Lanthanide series 59 62 63 65 67 69 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 13 1412 1401 14424 149 1630 16 150 p 94 151.9 aenicum 15725 158.99 Da n 97 1645 1676 173.04 ** Actinide series anun 92 89 90 91 93 95 96 98 99 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 24 214 238. p4 psa Select one: O a. CH3CH2CH2CH2NH2 a. Ob. (CH3)3COH O . CH3CH2CH2CH2OH d. (CH3)3CNH2 е. CH3CH2CH2CH2CH3
Which of the following compounds has the highest boiling point? hydogan 2 H Не 1009 hum 4.000 bendm bon carton rogen Oygen fne neon 5 B 3 10 Li Be N F Ne 6941 sodum 12011 slcon 14 14007 phonpone 15 15999 sultur 16 20.10 magnestm 12 al m 13 chiorine argon 11 17 18 Na Mg Al Si P S ci Ar 2430 ca 20 220 354 trome ndm 21 va m 23 pmanm 32 Mankm mongane 25 cobal 27 rkel krypn 36 polassum opper 19 22 24 26 28 29 30 31 33 34 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 40.0 4.0 am 7241 entm 44 palam 46 she cadam 48 antinony 51 utn 52 edine 53 won 37 38 39 40 41 42 43 45 47 49 50 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Хе sor 1212 casm 55 hahm 72 1124 meny 80 han 81 1260 aste 85 aon 86 bartm ngtm Iad 56 57-70 71 73 74 75 76 77 78 79 82 83 84 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 13291 anm 87 16.21 200 wtorgum 106 hassim 108 metnertum unum unuunm 110 bohrum 88 89-102 103 104 105 107 109 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uug lantham 57 centum 58 neodmtm 60 promettm 61 dypm 66 tum 68 st m 70 samanm europum gadoam 64 terbm *Lanthanide series 59 62 63 65 67 69 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 13 1412 1401 14424 149 1630 16 150 p 94 151.9 aenicum 15725 158.99 Da n 97 1645 1676 173.04 ** Actinide series anun 92 89 90 91 93 95 96 98 99 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 24 214 238. p4 psa Select one: O a. CH3CH2CH2CH2NH2 a. Ob. (CH3)3COH O . CH3CH2CH2CH2OH d. (CH3)3CNH2 е. CH3CH2CH2CH2CH3
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.40PAE: 8.40 Which of the following compounds would be expected to form intermolecular hydrogen bonds in the...
Related questions
Question
Transcribed Image Text:15.00
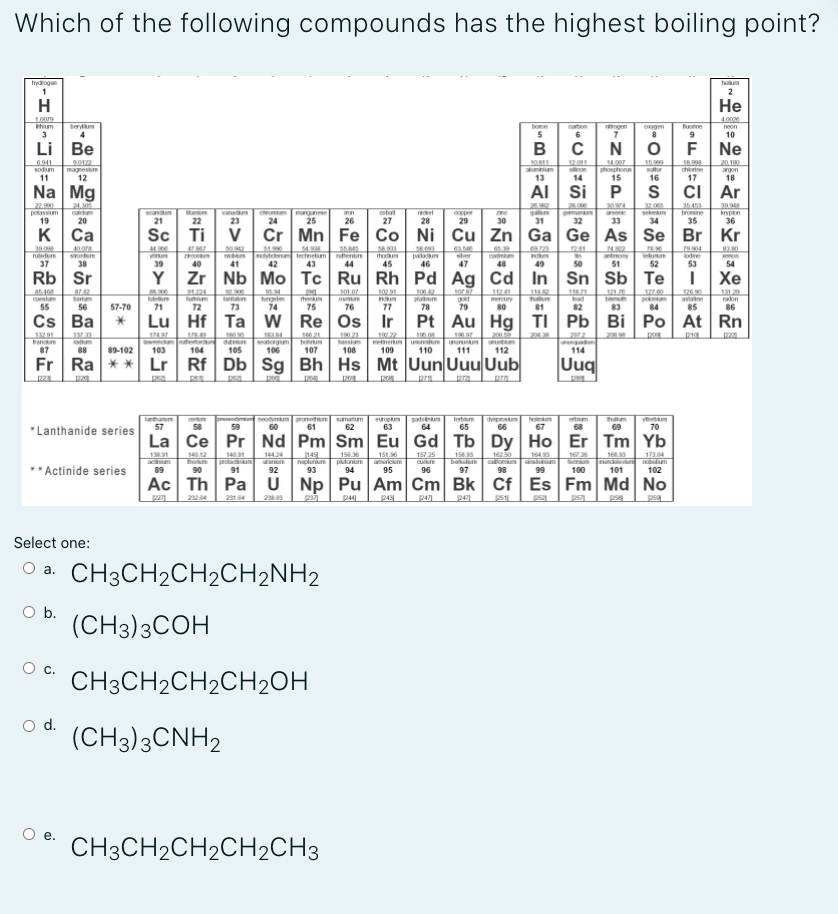
Which of the following compounds has the highest boiling point?
hydogan
hun
2
H
Не
4.00
1009
hum
berylum
bon
caton
roge
ORygen
fine
neon
5
В
3
10
Li Be
N
F
Ne
691
sodum
magnestm
12
a m
13
12011
son
14.00
phonphone
15
20.180
argon
18
sur
chrne
11
14
16
17
Na Mg
AI Si P
ci| Ar
35453
22.0
polassm
19
32.0
sem
21.30
Manm
22
vanam
23
comim nganse
24
25
cebalt
27
rkel
gmankum
32
tromne
krypn
36
anm
on
copper
20
21
26
28
29
30
31
33
34
35
к Са
Sc Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
241
nuidum
37
Montum
38
ytde Inctnetum
42
hentum
44
palaum
46
sher
cam
48
antnony
51
odine
53
on
39
40
41
43
45
47
49
50
52
54
Rb Sr
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te
Хе
or
cas
55
bartum
56
11241
merary
80
han
81
121.2
eth
83
12700
poknm
84
1260
aste
85
radon
86
ad
57-70
71
72
73
74
75
76
77
78
79
82
Cs Ba
Lu Hf Ta w Re Os Ir
Pt Au Hg
TI Pb Bi Po At Rn
13291
200
seaborgum
106
behrm
107
hasm
108
memertum uuntum unu m
110
randum
ditrm
87
88
89-102
103
104
105
109
111
112
114
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
pra
meodymum
60
gad m
64
dyeposm
66
y m
70
lantha
promethum
erom
63
samanum
terbm
homm
entm
57
58
59
61
62
65
67
68
69
*Lanthanide series
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
1381
14012
hon
90
14091
prota m
91
144.24
149
DHctunum
93
150.36
ponu
94
151.90
157.25
158.99
Daun
97
16250
164.5
16726
1734
** Actinide series
anun
92
89
95
96
98
99
100
101
102
Ac Th Pa
U Np Pu Am Cm Bk Cf Es Fm Md No
2324
231.4
238.0
ps
Select one:
Oa.
CH3CH2CH2CH2NH2
Ob.
(CH3)3COH
c.
CH3CH2CH2CH2OH
d.
(CH3)3CNH2
e.
CH3CH2CH2CH2CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
