Which of the following identifies the metal that is less easily oxidized, Al or Cu, and provides appropriate evidence? Aluminum, because aluminum metal loses electrons and copper ions gain electrons. Aluminum, because aluminum ions gain electrons and copper metal loses electrons. Copper, because copper metal loses electrons and aluminum ions gain electrons. Copper, because copper ions gain electrons and aluminum metal loses electrons.
Which of the following identifies the metal that is less easily oxidized, Al or Cu, and provides appropriate evidence? Aluminum, because aluminum metal loses electrons and copper ions gain electrons. Aluminum, because aluminum ions gain electrons and copper metal loses electrons. Copper, because copper metal loses electrons and aluminum ions gain electrons. Copper, because copper ions gain electrons and aluminum metal loses electrons.
Chapter5: Ground Rules Of Metabolism
Section: Chapter Questions
Problem 1SA: Which of the following statements is not correct? a. Energy cannot be created or destroyed. b....
Related questions
Question
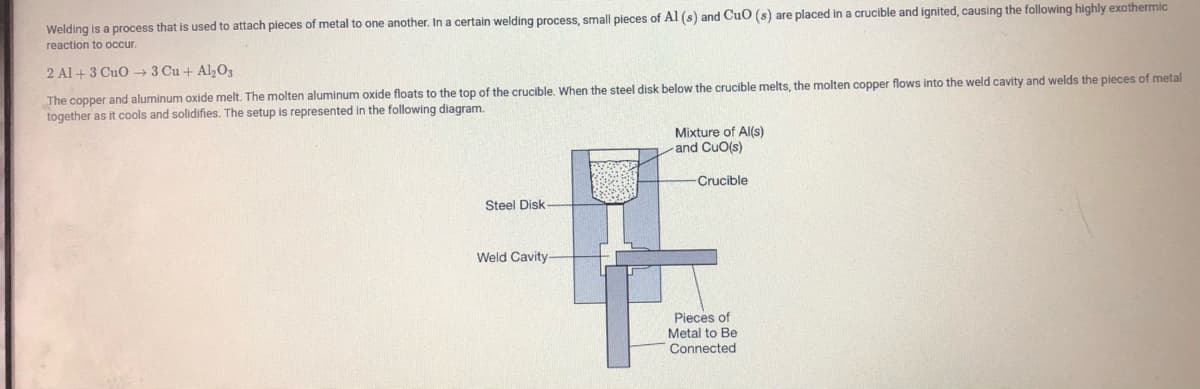
Transcribed Image Text:Welding is a process that
used to attach pieces of metal to one another. In a certain welding process, small pieces of Al (s) and CuO (s) are placed in a crucible and ignited, causing the following highly exothermic
reaction to occur.
2 Al + 3 CuO → 3 Cu + Al,0,
The copper and aluminum oxide melt. The molten aluminum oxide floats to the top of the crucible. When the steel disk below the crucible melts, the molten copper flows into the weld cavity and welds the pieces of metal
together as it cools and solidifies. The setup is represented in the following diagram.
Mixture of Al(s)
and CuO(s)
Crucible
Steel Disk
Weld Cavity
Pieces of
Metal to Be
Connected

Transcribed Image Text:Which of the following identifies the metal that is less easily oxidized, Al or Cu, and provides appropriate evidence?
Aluminum, because aluminum metal loses electrons and copper ions gain electrons.
Aluminum, because aluminum ions gain electrons and copper metal loses electrons.
Copper, because copper metal loses electrons and aluminum ions gain electrons.
Copper, because copper ions gain electrons and aluminum metal loses electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Human Heredity: Principles and Issues (MindTap Co…
Biology
ISBN:
9781305251052
Author:
Michael Cummings
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning


Human Heredity: Principles and Issues (MindTap Co…
Biology
ISBN:
9781305251052
Author:
Michael Cummings
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning