Which of the following is NOT a property of gases? Select one: O a. Gases are easy to compress. O b. The pressure exerted by a gas on its container is negligible. O c. Gases expand to occupy whatever volume is available. O d. Gases easily diffuse into one another. O e. Gases are described in terms of their temperature, pressure, volume, and number of moles of gas present.
Which of the following is NOT a property of gases? Select one: O a. Gases are easy to compress. O b. The pressure exerted by a gas on its container is negligible. O c. Gases expand to occupy whatever volume is available. O d. Gases easily diffuse into one another. O e. Gases are described in terms of their temperature, pressure, volume, and number of moles of gas present.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 157CP
Related questions
Question
100%
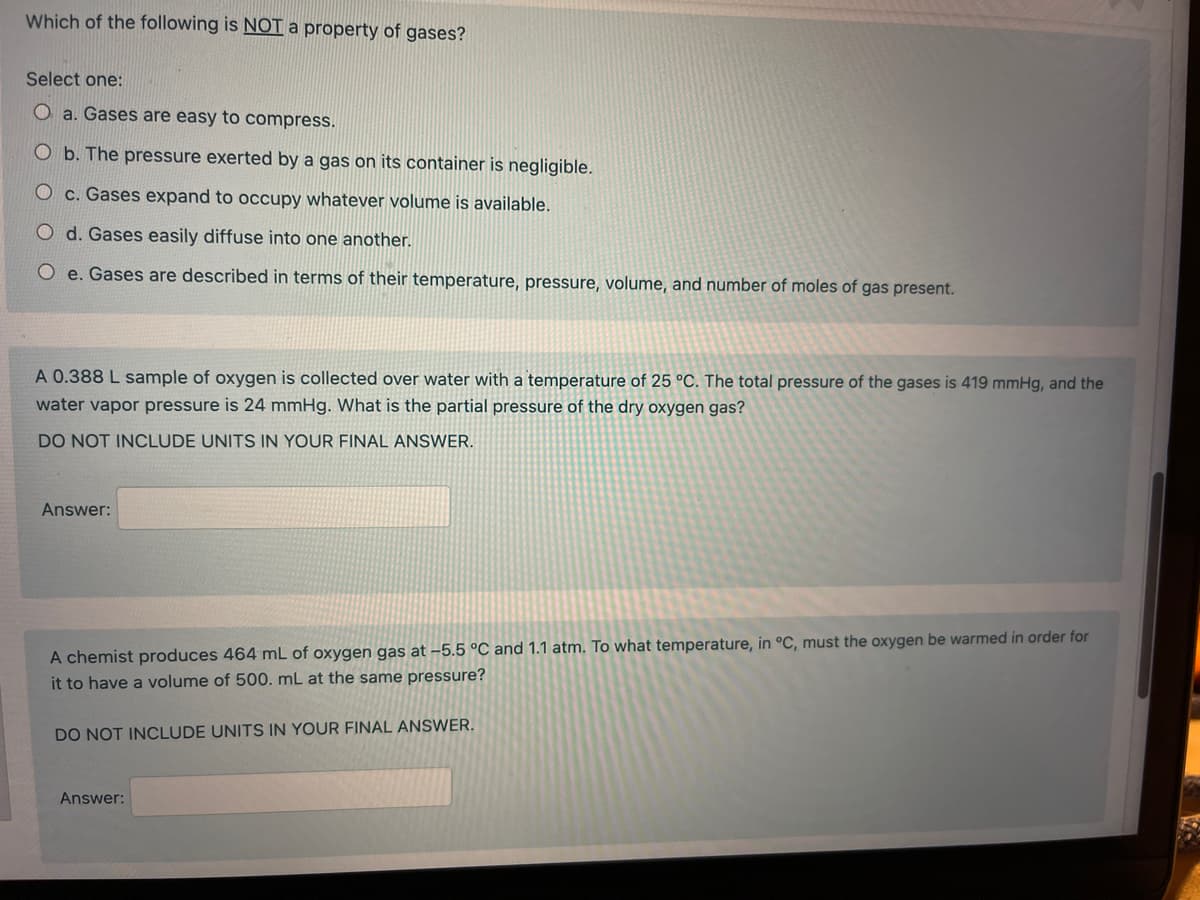
Transcribed Image Text:Which of the following is NOT a property of gases?
Select one:
O a. Gases are easy to compress.
O b. The pressure exerted by a gas on its container is negligible.
O c. Gases expand to occupy whatever volume is available.
O d. Gases easily diffuse into one another.
Oe. Gases are described in terms of their temperature, pressure, volume, and number of moles of gas present.
A 0.388 L sample of oxygen is collected over water with a temperature of 25 °C. The total pressure of the gases is 419 mmHg, and the
water vapor pressure is 24 mmHg. What is the partial pressure of the dry oxygen gas?
DO NOT INCLUDE UNITS IN YOUR FINAL ANSWER.
Answer:
A chemist produces 464 mL of oxygen gas at -5.5 °C and 1.1 atm. To what temperature, in °C, must the oxygen be warmed in order for
it to have a volume of 500. mL at the same pressure?
DO NOT INCLUDE UNITS IN YOUR FINAL ANSWER.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning