Chapter21: Synthesis Of N-butyl Bromide And T-pentyl Chloride
Section: Chapter Questions
Problem 1aQ
Related questions
Question
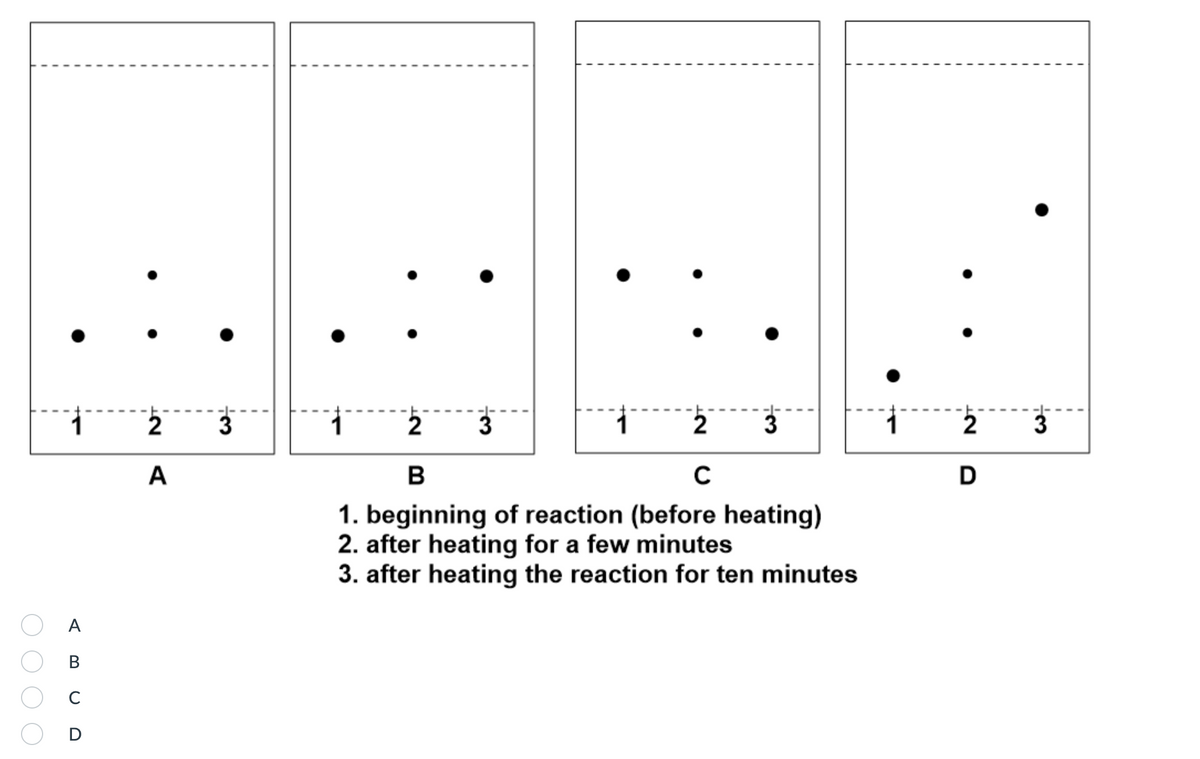
Question 6: Which of the following plates represents the course of the reaction from beginning to end, assuming a 100% yield?
Transcribed Image Text:1
2
3
2
3
2
3
1
2
3
A
1. beginning of reaction (before heating)
2. after heating for a few minutes
3. after heating the reaction for ten minutes
A
В
C
D
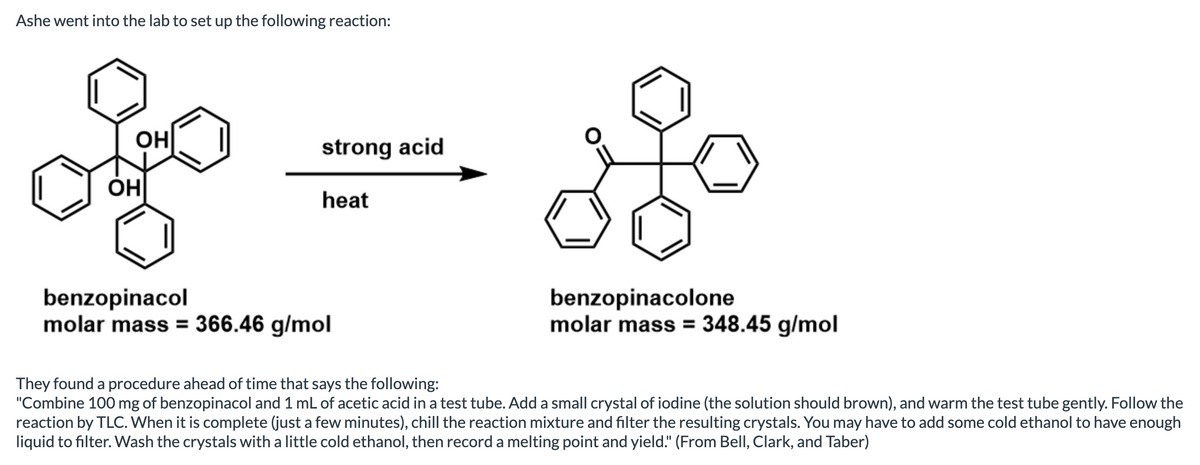
Transcribed Image Text:Ashe went into the lab to set up the following reaction:
OH
strong acid
OH
heat
benzopinacol
molar mass = 366.46 g/mol
benzopinacolone
molar mass = 348.45 g/mol
They found a procedure ahead of time that says the following:
"Combine 100 mg of benzopinacol and 1 mL of acetic acid in a test tube. Add a small crystal of iodine (the solution should brown), and warm the test tube gently. Follow the
reaction by TLC. When it is complete (just a few minutes), chill the reaction mixture and filter the resulting crystals. You may have to add some cold ethanol to have enough
liquid to filter. Wash the crystals with a little cold ethanol, then record a melting point and yield." (From Bell, Clark, and Taber)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT