Which of the following salts is most soluble? Ca2(PO4)3 - Ksp = 2.1 10-33 AgCl - Ksp = 1.8 * 10-10 Pbl2 - Ksp = 8.7 10-9 Ga(OH)3 - Ksp = 8.6* 10-9
Which of the following salts is most soluble? Ca2(PO4)3 - Ksp = 2.1 10-33 AgCl - Ksp = 1.8 * 10-10 Pbl2 - Ksp = 8.7 10-9 Ga(OH)3 - Ksp = 8.6* 10-9
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.4: Solubility Of Salts
Problem 4RC: 4. What is the solubility of PbSO4 in water at 25°C if the solution already contains 0.25 M...
Related questions
Question
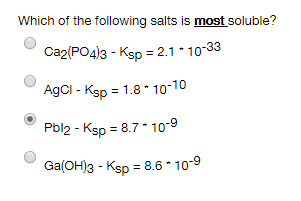
Transcribed Image Text:Which of the following salts is most soluble?
Ca2(PO4)3 - Ksp = 2.1 10-33
AgCl - Ksp = 1.8 * 10-10
Pbl2 - Ksp = 8.7 10-9
Ga(OH)3 - Ksp = 8.6* 10-9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole