Which of the following statements about entropy is true? Select one: O a. The cntropy of a substance increases with a decrease in the number of moles. O b. The entropy of a substance will increase on going from a solid to a liquid to a gas. O C. The entropy of any substance increases as the temperature is lowered O d. The entr opy of a gas decreases with an increase in volume. O e. The entropy of a gas is independent of the volume of the gas.
Which of the following statements about entropy is true? Select one: O a. The cntropy of a substance increases with a decrease in the number of moles. O b. The entropy of a substance will increase on going from a solid to a liquid to a gas. O C. The entropy of any substance increases as the temperature is lowered O d. The entr opy of a gas decreases with an increase in volume. O e. The entropy of a gas is independent of the volume of the gas.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.47PAE: The reaction CO2(g)+H2(g)CO(g)+H2O(g) is not spontaneous at room temperature but becomes spontaneous...
Related questions
Question
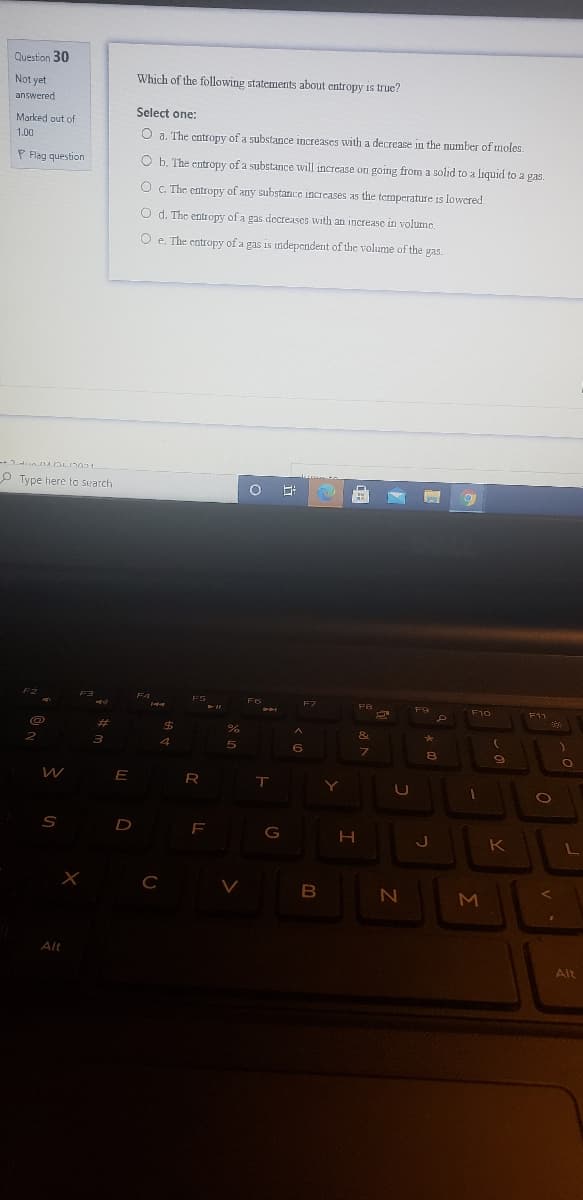
Transcribed Image Text:Question 30
Not yet
Which of the following statements about entropy is true?
answered
Select one:
Marked out of
1.00
O a. The entropy of a substance increases with a decrease in the number of moles.
P Flag question
O b. The entropy of a substance will increase on going from a solid to a liquid to a gas.
O C. The entropy of any substance increases as the temperature is lowered
O d. The entIopy of a gas dccreases with an increase in volume.
O e. The catropy of a gas is independent of the volume of the gas.
P Type here to search
F2
F3
F4
ES
F8
F9
F10
F11
%23
%24
&
E
R
D
G
K
C
M
Alt
Alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning