Which of the following statements regarding complexometric titrations is incorrect ? OIn the pH range 3-10 the predominant species are H2Y2 and HY3- O The higher the ammonia concentration, the less sharper the end point for the EDTA-Zn2* complex. O EDTA is a hexaprotic acid. O a4 value is proportional to the pH value. O The larger the formation constant for the EDTA-metal complex, the sharper the end point.
Which of the following statements regarding complexometric titrations is incorrect ? OIn the pH range 3-10 the predominant species are H2Y2 and HY3- O The higher the ammonia concentration, the less sharper the end point for the EDTA-Zn2* complex. O EDTA is a hexaprotic acid. O a4 value is proportional to the pH value. O The larger the formation constant for the EDTA-metal complex, the sharper the end point.
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.25QAP
Related questions
Question
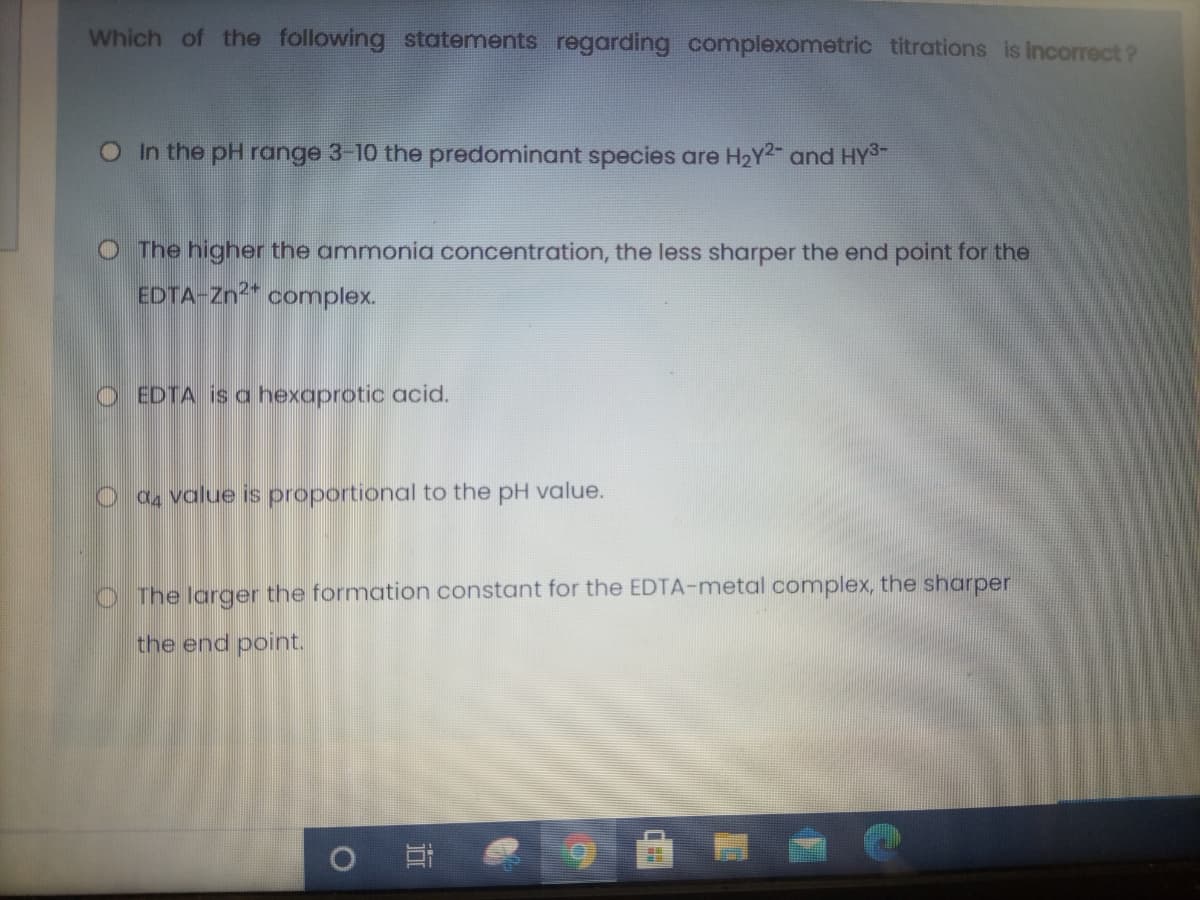
Transcribed Image Text:Which of the following statements regarding complexometric titrations is incorrect ?
OIn the pH range 3-10 the predominant species are H2Y2 and HYS-
O The higher the ammonia concentration, the less sharper the end point for the
EDTA-Zn2* complex.
O EDTA is a hexaprotic acid.
O a value is proportional to the pH value.
O The larger the formation constant for the EDTA-metal complex, the sharper
the end point.
五
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning