Which statement about isotopes is false? O Radioisotopes are unstable and spontaneously emit radiation. Isotopes have virtually the same chemical reactivity. Deuterium is an isotope of hydrogen containing one proton and two neutrons. Isotopes have the same atomic number but not the same atomic weight. Isotopes vary in the number of neutrons but not the number of protons.
Which statement about isotopes is false? O Radioisotopes are unstable and spontaneously emit radiation. Isotopes have virtually the same chemical reactivity. Deuterium is an isotope of hydrogen containing one proton and two neutrons. Isotopes have the same atomic number but not the same atomic weight. Isotopes vary in the number of neutrons but not the number of protons.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter3: Atomic Structures And The Periodic Table
Section: Chapter Questions
Problem 3.37EP
Related questions
Question
please see attached
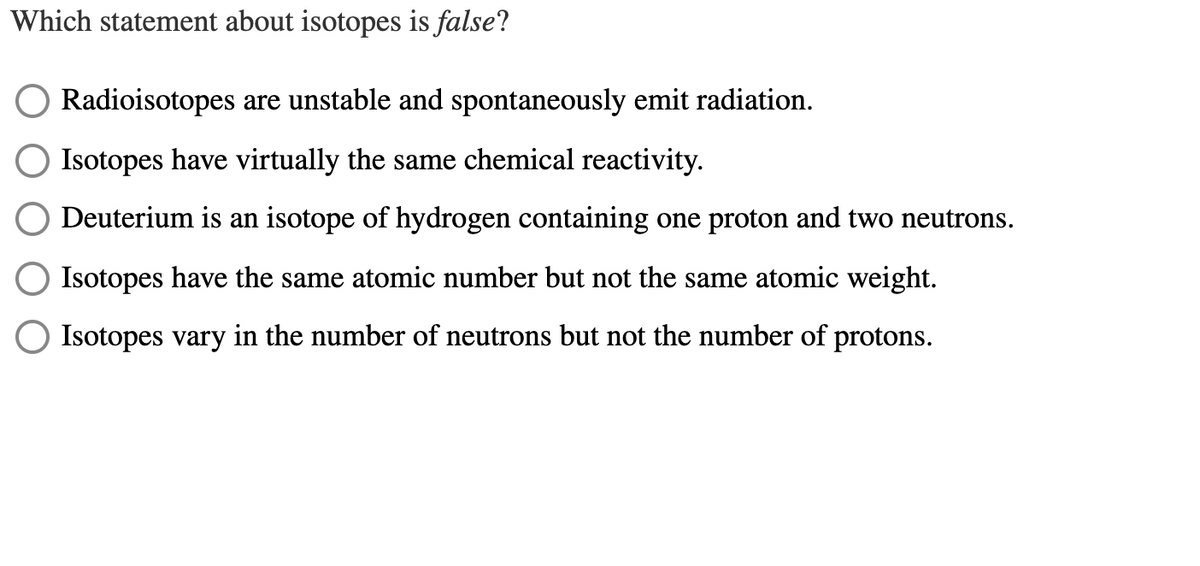
Transcribed Image Text:Which statement about isotopes is false?
Radioisotopes are unstable and spontaneously emit radiation.
Isotopes have virtually the same chemical reactivity.
Deuterium is an isotope of hydrogen containing one proton and two neutrons.
Isotopes have the same atomic number but not the same atomic weight.
Isotopes vary in the number of neutrons but not the number of protons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
