While trying to synthesise product A following the reaction scheme shown below, the desired product was never obtained. Instead an unwanted product B was obtained in 100% yield. Provide the detailed mechanism of how this product formed and suggest a new synthesis, starting from the given starting material to obtain product A. 1. Mg 2.0 3. H₂O* emo A OH 0% yield Joh B 100% yield
While trying to synthesise product A following the reaction scheme shown below, the desired product was never obtained. Instead an unwanted product B was obtained in 100% yield. Provide the detailed mechanism of how this product formed and suggest a new synthesis, starting from the given starting material to obtain product A. 1. Mg 2.0 3. H₂O* emo A OH 0% yield Joh B 100% yield
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.63P
Related questions
Question
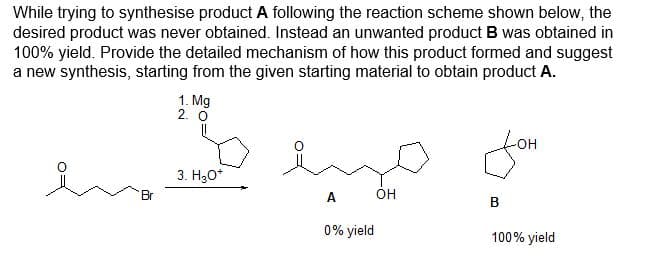
Transcribed Image Text:While trying to synthesise product A following the reaction scheme shown below, the
desired product was never obtained. Instead an unwanted product B was obtained in
100% yield. Provide the detailed mechanism of how this product formed and suggest
a new synthesis, starting from the given starting material to obtain product A.
1. Mg
2. O
en 10
3. H₂O*
Br
ميا
A
0% yield
fom
B
100% yield
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
