Without doing any calculations, determine the sign of AS, for each chemical reaction. a. Mg(s) + Cl(g) b. 2 H,S(g) + 3 O,(g) c. 2 0;(g) 3 0:(g) d. HCl(g) + NH;(g) NH,CI(s) MgCl,(s) - 2 H;O(g) + 2 SO2(g)
Without doing any calculations, determine the sign of AS, for each chemical reaction. a. Mg(s) + Cl(g) b. 2 H,S(g) + 3 O,(g) c. 2 0;(g) 3 0:(g) d. HCl(g) + NH;(g) NH,CI(s) MgCl,(s) - 2 H;O(g) + 2 SO2(g)
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter18: Thermodynamics And Equilibrium
Section: Chapter Questions
Problem 18.73QP
Related questions
Question
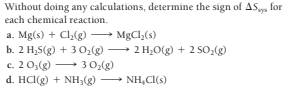
Transcribed Image Text:Without doing any calculations, determine the sign of AS, for
each chemical reaction.
a. Mg(s) + Cl(g)
b. 2 H,S(g) + 3 O,(g)
c. 2 0;(g) 3 0:(g)
d. HCl(g) + NH;(g) NH,CI(s)
MgCl,(s)
- 2 H;O(g) + 2 SO2(g)
Expert Solution
Introduction
Entropy is the unit of randomness. Change in entropy i.e can be calculated by subtracting the entropy of reactants by the entropy of products.
More the number of moles present,more will be the entropy.
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning