write molecular equation for the following precipitation reaction. Copper(II) chloride and sodium suifde are dissolved in water. (Use the lowest possible coefficients. Be sure to specity states such as (aq) or (s). If a box is not needed, leave it blank.) CuCl, (ng) + Na, 8(6) - Cus(o) + 2NaCl(ag) CuCl, (aq) + NagS(aq) CuS(a) + 2NACI(aq) Write net ionic equation for this reaction. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it bla
write molecular equation for the following precipitation reaction. Copper(II) chloride and sodium suifde are dissolved in water. (Use the lowest possible coefficients. Be sure to specity states such as (aq) or (s). If a box is not needed, leave it blank.) CuCl, (ng) + Na, 8(6) - Cus(o) + 2NaCl(ag) CuCl, (aq) + NagS(aq) CuS(a) + 2NACI(aq) Write net ionic equation for this reaction. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it bla
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 81GQ: Balance equations for these reactions that occur in aqueous solution, and then classify each as a...
Related questions
Question
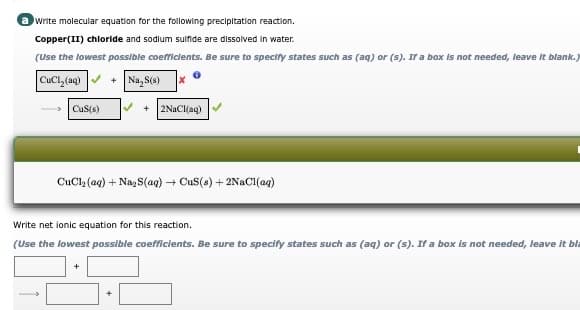
Transcribed Image Text:write molecular equation for the following precipitation reaction.
Copper(II) chloride and sodium sulfide are dissolved in water.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave It blank.)
CuCl, (aq) v + Na,S(e)
- CuS(s)
+ 2NaCl(aq)
CuCla (aq) + Nag S(aq) + Cus(s) + 2NAC1(aq)
Write net ionic equation for this reaction.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it bla
Expert Solution
Step 1
CuCl2 and Na2S are dissolved in water. So, they represented as (aq).
Molecular equation is
CuCl2 (aq) + Na2S (aq) ---------> CuS (s) + 2 NaCl (aq)
According to solubility rules,
Sulfides are insoluble except 1st group and ammonium ions. So, CuS is insoluble.
So, precipitate is formed in the reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning