- Write the chemical equation(s) for each part of the experiment, as outlined in Equation 1. (0) Part I Cuole)de.I (a) Part I (b) Part II Cu(s) (Cu(H,O),* (aq) Cu(OH),(s) CuO(s) (d) Part IV (e) Part V (Cu(H,O),* (aq) Cu(s) dihoeuls vile
- Write the chemical equation(s) for each part of the experiment, as outlined in Equation 1. (0) Part I Cuole)de.I (a) Part I (b) Part II Cu(s) (Cu(H,O),* (aq) Cu(OH),(s) CuO(s) (d) Part IV (e) Part V (Cu(H,O),* (aq) Cu(s) dihoeuls vile
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 4RQ: Consider the equation G = -nF. What are the four terms in this equation? Why does a minus sign...
Related questions
Question

Transcribed Image Text:Ford
VOL
3. Write the chemical equation(s) for each part of the experiment, as outlined in Equation 1.
(a) Part I
Cu(s)
(Cu(H,O),* (aq)
12+
(b) Part II
(c) Part III
Cu(OH),(s)
CuO(s)
(d) Part IV
[Cu(H,O),* (aq)
12+
(e) Part V
Cu(s)
bodl amul s i OVH diw lo noitsbixo art tuo yuso teurm uoy dw
a Ternary Mixture
Expert Solution
Step 1
(a)
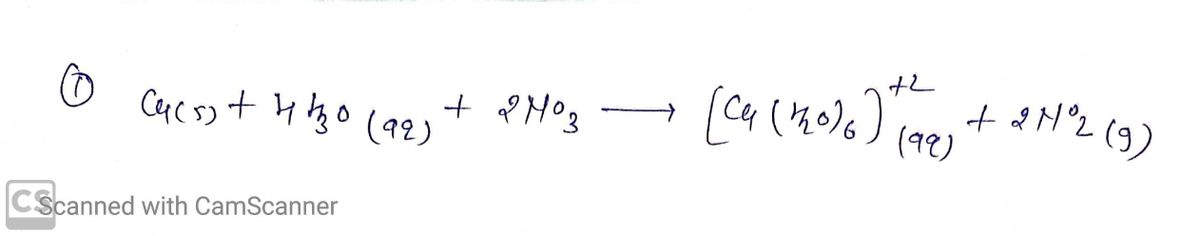 This reaction actually occurs in 2 steps. In the first step, the copper (red-brown)
This reaction actually occurs in 2 steps. In the first step, the copper (red-brown)
is oxidized to copper(II) ions by nitrate ions that the addition of nitric acid (HNO3)
suppliesAt the same time, the Nitrate ions (NO3-) are reduced to nitrogen(IV) oxide
(NO2), a brown gas. When all of the copper has been oxidized we perform the second
step by diluting the reaction mixture (RM) with water. The addition of H2O produces
hexaaquocopper(II) ions, [Cu(H2O)6]2+, a blue solution.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning