Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 72QRT
Related questions
Question
Transcribed Image Text:19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
4
K
Са
Sc
Ti
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb
Мо
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
Хе
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
6.
Cs
Ва
La
Hf
Ta
W
Re
Os
Pt
Au
Hg
TI
Pb
Bi
Ро
At
Rn
87
88
89
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
7
Fr
Ra
Ac
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Nh
FI
Mc
Lv
Ts
Og
f2
f3
f5
f6
f8
f10
f11
f12
f13
f14
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Но
Er
Tm
Yb
Lu
90
91
92
93
94
95
96
97
98
99
100
101
102
103
7
Th
Ра
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
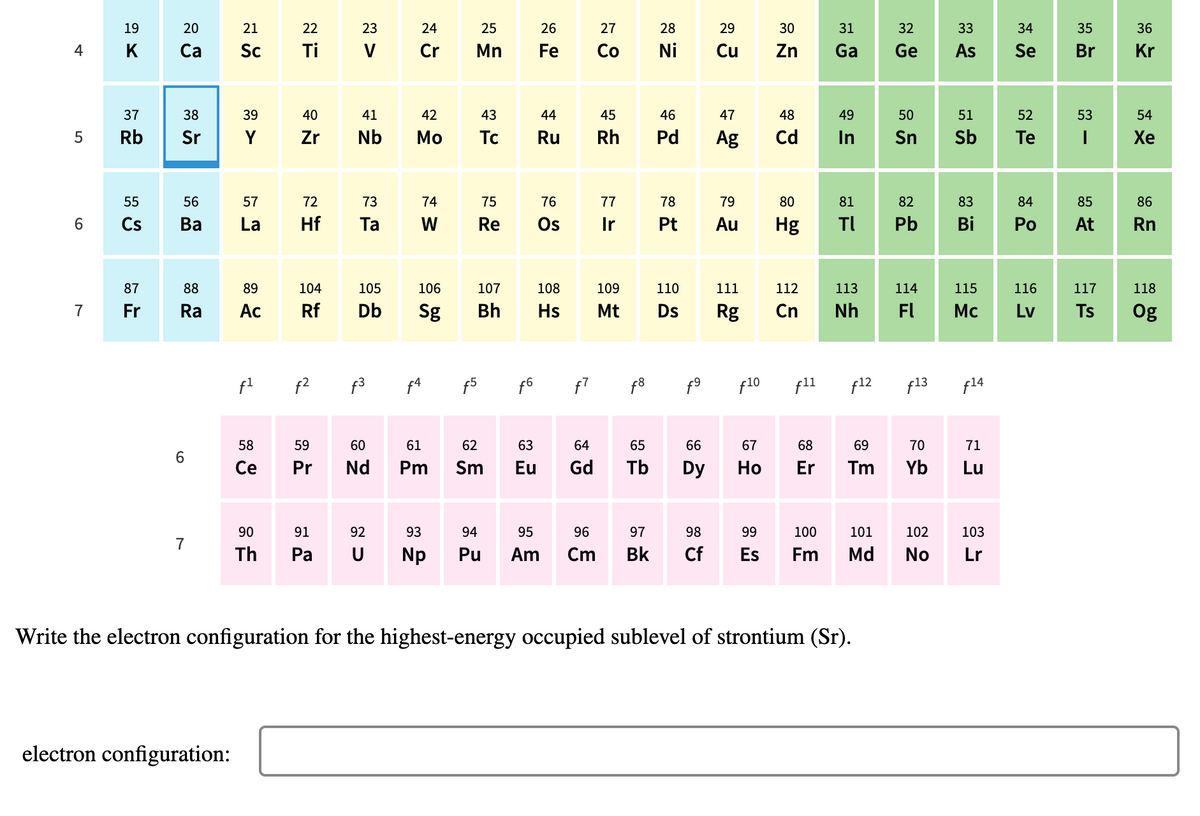
Write the electron configuration for the highest-energy occupied sublevel of strontium (Sr).
electron configuration:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning