Chapter29: Mass Spectrometry
Section: Chapter Questions
Problem 29.12QAP
Related questions
Question
Write the objective of separation of 2-Butanone from Toluene by distillation
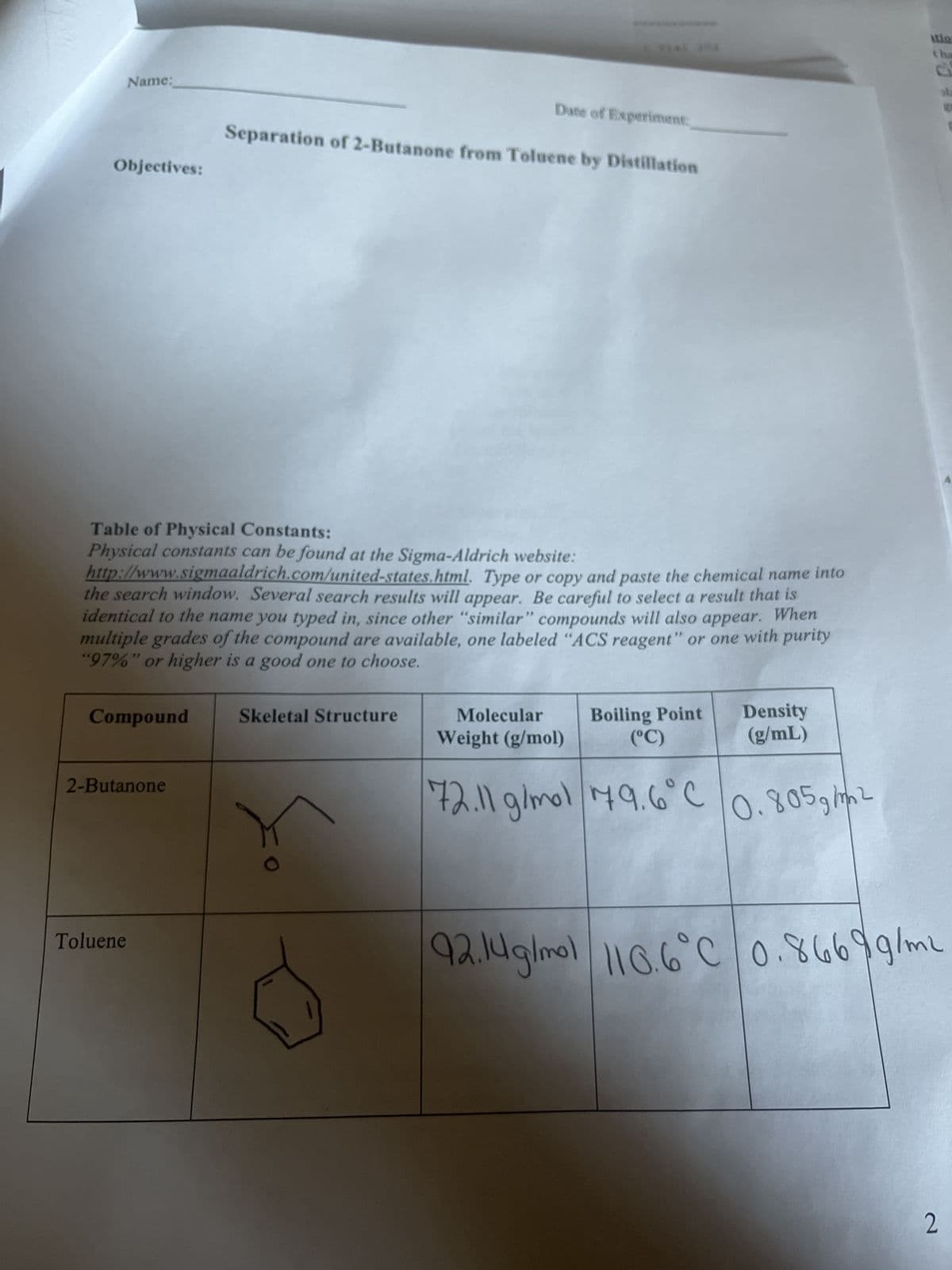
Transcribed Image Text:Name:
Objectives:
Compound
2-Butanone
Toluene
Table of Physical Constants:
Physical constants can be found at the Sigma-Aldrich website:
http://www.sigmaaldrich.com/united-states.html. Type or copy and paste the chemical name into
the search window. Several search results will appear. Be careful to select a result that is
identical to the name you typed in, since other "similar" compounds will also appear. When
multiple grades of the compound are available, one labeled "ACS reagent" or one with purity
"97%" or higher is a good one to choose.
*Y*** 200
Date of Experiment:
Separation of 2-Butanone from Toluene by Distillation
Skeletal Structure
Boiling Point Density
(°C)
(g/mL)
Molecular
Weight (g/mol)
72.11 g/mol 79.6°C
0.805g/m2
itio
the
92.14g/mol 110.6°C 0.8669g/mL
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning