www Combustion of glucose (C H20) is the main source of energy for animal cells: AG (37 °C) =-2872. kJ 6 CO2(g)+ 6 H2O() CH1206(s) +602(g) rxn This energy is generally stored as ATP (adenosine triphosphate) molecules, which can release it when convenient by hydrolysis ("water-assisted in biochemistry: decomposition") into ADP (adenosine diphosphate) molecules and a phosphate anion, often given the symbol P = -35 to 70 kJ хn AG ADP (aq) +P, (aq). ATP(aq) The actual amount of free energy released by the hydrolysis of ATP varies, depending on the exact conditions inside the cell. Suppose under certain conditions hydrolysis of ATP actually releases -44.0 kJ/mol. Calculate the maximum number of ATPS that could be created from ADPS and P, by the combustion of a molecule of glucose. Your answer must be a whole number. ? Check Explanation 2019 McGraw-Hill Education. All Rights Reserved. Terms o Anse End Home Eso F12 F11 F10 Fo F8 F7 F6 F5 F4 F3 FnLock & % X
www Combustion of glucose (C H20) is the main source of energy for animal cells: AG (37 °C) =-2872. kJ 6 CO2(g)+ 6 H2O() CH1206(s) +602(g) rxn This energy is generally stored as ATP (adenosine triphosphate) molecules, which can release it when convenient by hydrolysis ("water-assisted in biochemistry: decomposition") into ADP (adenosine diphosphate) molecules and a phosphate anion, often given the symbol P = -35 to 70 kJ хn AG ADP (aq) +P, (aq). ATP(aq) The actual amount of free energy released by the hydrolysis of ATP varies, depending on the exact conditions inside the cell. Suppose under certain conditions hydrolysis of ATP actually releases -44.0 kJ/mol. Calculate the maximum number of ATPS that could be created from ADPS and P, by the combustion of a molecule of glucose. Your answer must be a whole number. ? Check Explanation 2019 McGraw-Hill Education. All Rights Reserved. Terms o Anse End Home Eso F12 F11 F10 Fo F8 F7 F6 F5 F4 F3 FnLock & % X
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.86PAE: The enthalpy of vaporization for water is 40.65 kJ mol-1. As a design engineer for a project in a...
Related questions
Question
Calculate the maximum number of ATPs that could be created from ADP and PI. By the combustion of the
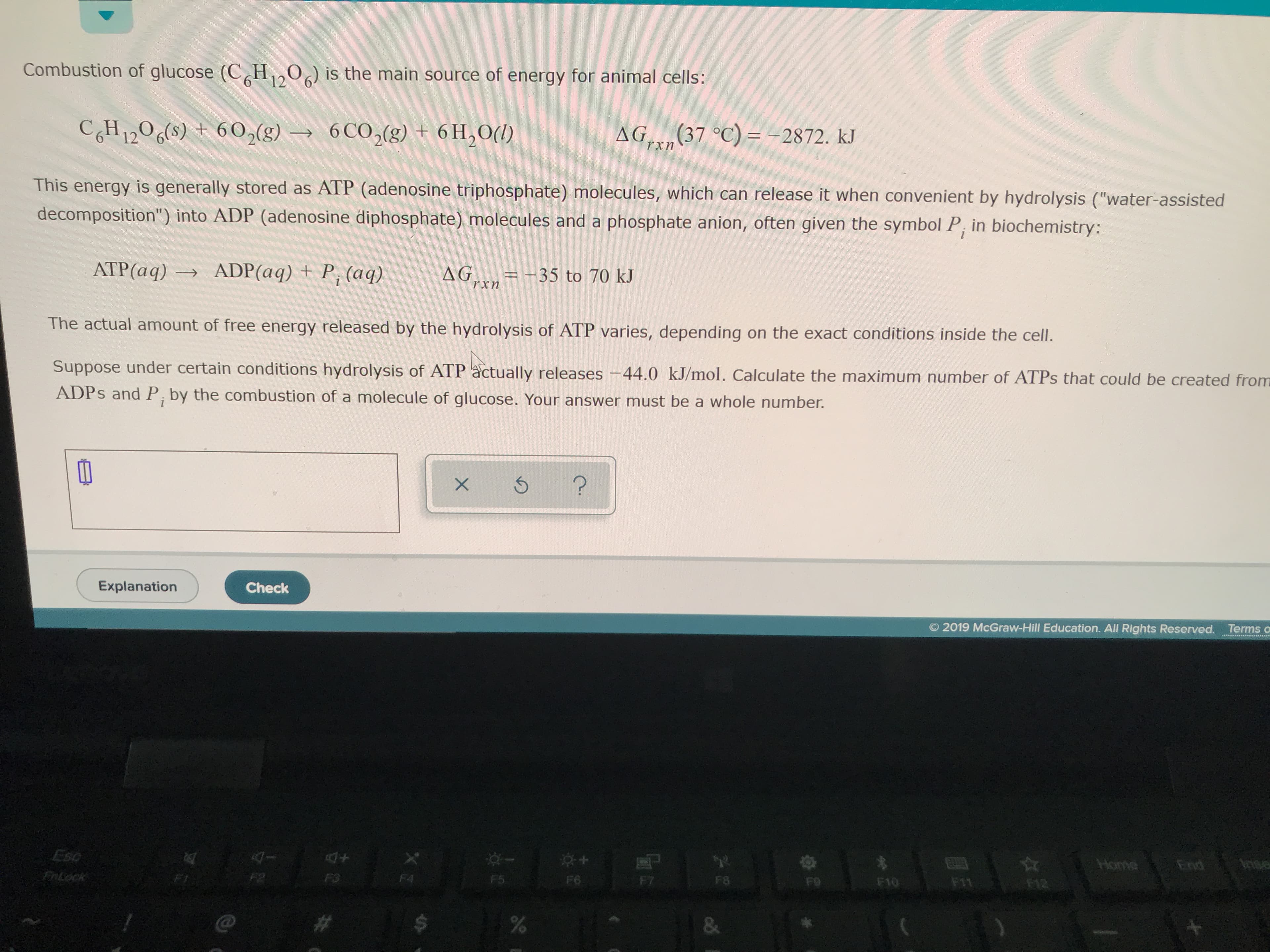
Transcribed Image Text:www
Combustion of glucose (C H20) is the main source of energy for animal cells:
AG (37 °C) =-2872. kJ
6 CO2(g)+ 6 H2O()
CH1206(s) +602(g)
rxn
This energy is generally stored as ATP (adenosine triphosphate) molecules, which can release it when convenient by hydrolysis ("water-assisted
in biochemistry:
decomposition") into ADP (adenosine diphosphate) molecules and a phosphate anion, often given the symbol P
= -35 to 70 kJ
хn
AG
ADP (aq) +P, (aq).
ATP(aq)
The actual amount of free energy released by the hydrolysis of ATP varies, depending on the exact conditions inside the cell.
Suppose under certain conditions hydrolysis of ATP actually releases -44.0 kJ/mol. Calculate the maximum number of ATPS that could be created from
ADPS and P, by the combustion of a molecule of glucose. Your answer must be a whole number.
?
Check
Explanation
2019 McGraw-Hill Education. All Rights Reserved. Terms o
Anse
End
Home
Eso
F12
F11
F10
Fo
F8
F7
F6
F5
F4
F3
FnLock
&
%
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning