WWW TAT Groups are numbered 3B to 12B from left to right across the periodic table for the transition metals only. Group 3B Sc Group 4B Ti Group 5B V Group 6B Cr Group 7B Mn Group 8B Fe Group 9B Co Group 10B Ni Group 11B Cu Group 12B Zn 1. Period 1, Group 1A 2. Period 1, Group 8A 3. Period 3, Group 3A 4. Period 2, Group 6A 5. Period 5, Group 2A 6. Period 4, Group 5A 7. Period 4, Group 8A 8. Period 3, Group 6A Element identification. Write the chemical symbol for the element at the given location on the periodic table of the elements. The location is written as "Period, Group". The chemical symbol for an element may be 1 or 2 letters. If 1 letter, it must be capitalized. If 2 letters, the first letter is capitalized and the second letter is lower case. LIVEWORKSHEETS 9. Period 4, Group 3B 10. Period 6, Group 6B 11. Period 5, Group 12B 12. Period 4, Group 7B 13. Period 5, Group. 11B 14. Period 6, Group 4B 15. Period 5, Group 5B 16. Period 4, Group 12B
WWW TAT Groups are numbered 3B to 12B from left to right across the periodic table for the transition metals only. Group 3B Sc Group 4B Ti Group 5B V Group 6B Cr Group 7B Mn Group 8B Fe Group 9B Co Group 10B Ni Group 11B Cu Group 12B Zn 1. Period 1, Group 1A 2. Period 1, Group 8A 3. Period 3, Group 3A 4. Period 2, Group 6A 5. Period 5, Group 2A 6. Period 4, Group 5A 7. Period 4, Group 8A 8. Period 3, Group 6A Element identification. Write the chemical symbol for the element at the given location on the periodic table of the elements. The location is written as "Period, Group". The chemical symbol for an element may be 1 or 2 letters. If 1 letter, it must be capitalized. If 2 letters, the first letter is capitalized and the second letter is lower case. LIVEWORKSHEETS 9. Period 4, Group 3B 10. Period 6, Group 6B 11. Period 5, Group 12B 12. Period 4, Group 7B 13. Period 5, Group. 11B 14. Period 6, Group 4B 15. Period 5, Group 5B 16. Period 4, Group 12B
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 25E: The average atomic masses of some elements may vary, depending upon the sources of their ores....
Related questions
Question
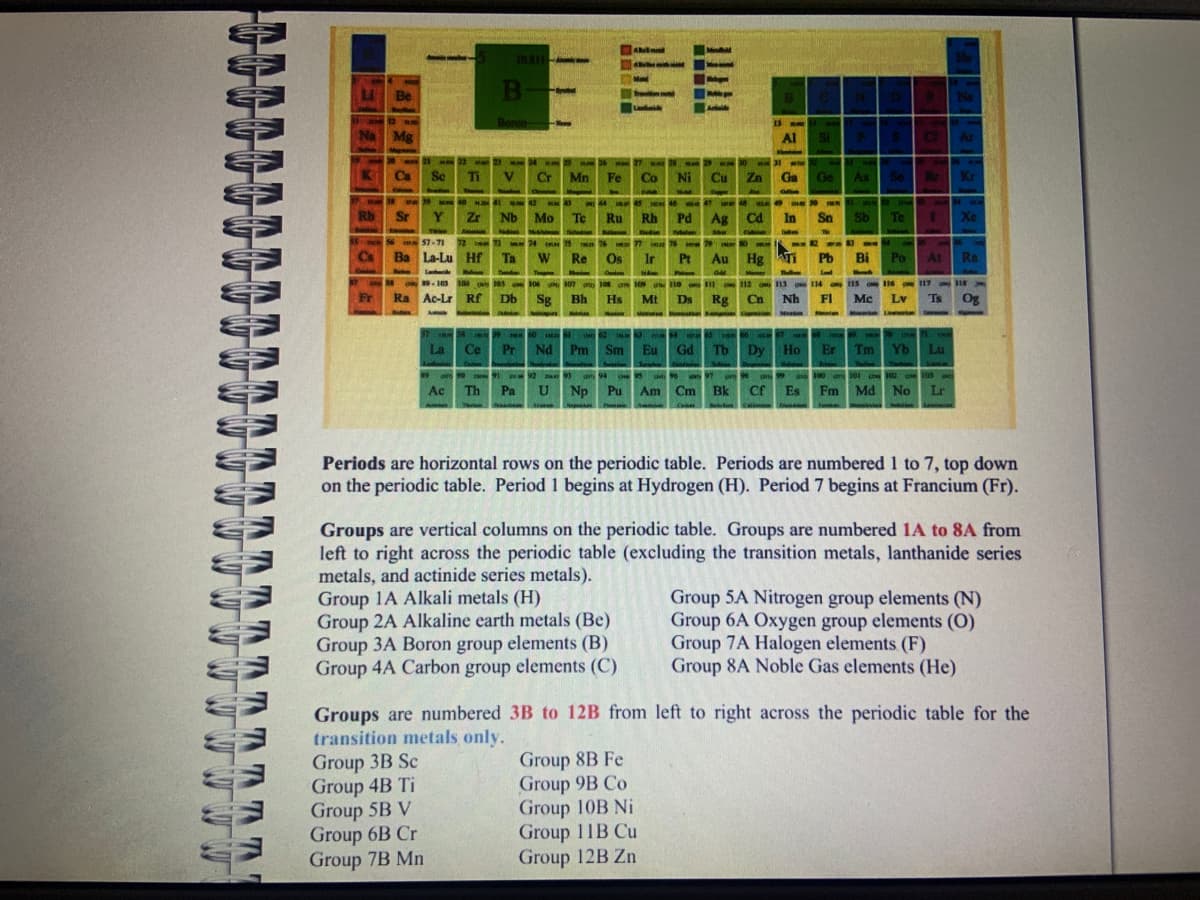
Transcribed Image Text:TAT
W
Na Mg
Rb
Be
Cs
wa
Sr
Se Ti
M40 HD
Zr
FEE
M
Drukkn d
Landare
10.811
B
Boron-
57-71 72
23
Ba La-Lu Hf Ta
Balen
Group 3B Sc
Group 4B Ti
Group 5B V
Group 6B Cr
Group 7B Mn
Nb Mo Te
Lasteride
99-103 104 105
Fr Ra Ac-Lr Rf Db
Cr Mn
24 25
Fe
Ru
W Re Os
27 wa
29 30
Co Ni Cu Zn
Eda
Rh
Pd
47
ar 99 91 92 93 un 94 10 95
Ac Th
106 107 108 109 110 111
Sg Bh Hs
Mt Ds Rg
1914
Ag Cd
13 AM
Al
77
Ir Pt Au Hg Ti
GM
Group 8B Fe
Group 9B Co
Group 10B Ni
Group 11B Cu
Group 12B Zn
Ga
In
112 113
Cn Nh
N 97 om 98
Pa U Np
Np Pu Am Cm Bk Cf Es
99
99
C
Si
Ge As Se Br Kr
50 NIE
Sn Sb Te I
Pb Bi
115
Mc
1 58 59 60 61 in 62 18D
64 65 66 67 we
THEOK
La
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
114
FI
Po
116
Lv
Lawri
CI
100
100 101 102
18e
At Rn
117 ON 118
118
INS
Xe
Ts Og
Lu
103
Es Fm Md No Lr
Periods are horizontal rows on the periodic table. Periods are numbered 1 to 7, top down
on the periodic table. Period 1 begins at Hydrogen (H). Period 7 begins at Francium (Fr).
Groups are vertical columns on the periodic table. Groups are numbered 1A to 8A from
left to right across the periodic table (excluding the transition metals, lanthanide series
metals, and actinide series metals).
Group 1A Alkali metals (H)
Group 2A Alkaline earth metals (Be)
Group 3A Boron group elements (B)
Group 4A Carbon group elements (C)
Groups are numbered 3B to 12B from left to right across the periodic table for the
transition metals only.
Group 5A Nitrogen group elements (N)
Group 6A Oxygen group elements (0)
Group 7A Halogen elements (F)
Group 8A Noble Gas elements (He)

Transcribed Image Text:TITT
Groups are numbered 3B to 12B from left to right across the periodic table for the
transition metals only.
Group 3B Sc
Group 4B Ti
Group SB V
Group 6B Cr
Group 7B Mn
Group 8B Fe
Group 9B Co
Group 10B Ni
Group 11B Cu
Group 12B Zn
1. Period 1, Group 1A
2. Period 1, Group 8A
3. Period 3, Group 3A
4. Period 2, Group 6A
5. Period 5, Group 2A
6. Period 4, Group 5A
7. Period 4, Group 8A
8. Period 3, Group 6A
Element identification. Write the chemical symbol for the element at the given location
on the periodic table of the elements. The location is written as "Period, Group". The
chemical symbol for an element may be 1 or 2 letters. If 1 letter, it must be capitalized. If
2 letters, the first letter is capitalized and the second letter is lower case.
LIVEWORKSHEETS
9. Period 4, Group 3B
10. Period 6, Group 6B
11. Period 5, Group 12B
12. Period 4, Group 7B
13. Period 5, Group. 11B
14. Period 6, Group 4B
15. Period 5, Group 5B
16. Period 4, Group 12B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning