You add the weighed KHP to a flask containing a 60mL of water rather than 50 mL of water. Explain. Your answer must contain a well-thought-out and clearly written explanation. b) The buret is still wet with water on the inside when you add your NaOH solution. Explain c) The KHP is wet when you weigh it. Explain. d) You titrate past the equivalence point by 0.50mL Answer a-d
You add the weighed KHP to a flask containing a 60mL of water rather than 50 mL of water. Explain. Your answer must contain a well-thought-out and clearly written explanation. b) The buret is still wet with water on the inside when you add your NaOH solution. Explain c) The KHP is wet when you weigh it. Explain. d) You titrate past the equivalence point by 0.50mL Answer a-d
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.42QAP
Related questions
Question
Will the calculated Molarity of NaOH be too high or too low or unaffected if the following happen: When you answer the question, consider how the situation affects the calculation of molarity of NaOH and moles NaOH, which is calculated from the mass of KHP. For each answer, you must supply a clear explanation for your answer. Often times, students will restate the facts rather than explaining why they chose the answer they chose.
a) You add the weighed KHP to a flask containing a 60mL of water rather than 50 mL of water. Explain. Your answer must contain a well-thought-out and clearly written explanation.
b) The buret is still wet with water on the inside when you add your NaOH solution. Explain
c) The KHP is wet when you weigh it. Explain.
d) You titrate past the equivalence point by 0.50mL
Answer a-d
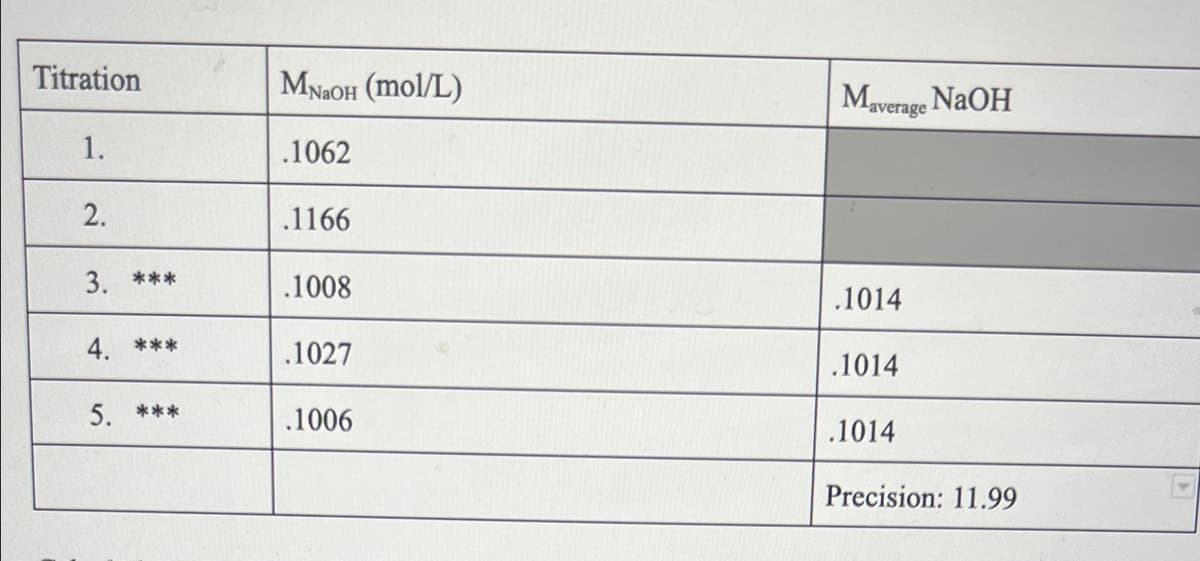
Transcribed Image Text:Titration
MNAOH (mol/L)
Maverage NAOH
1.
.1062
2.
.1166
3. ***
.1008
.1014
4. ***
.1027
.1014
5. ***
.1006
.1014
Precision: 11.99

Transcribed Image Text:a) You add the weighed KHP to a flask containing a 60mL of water rather than 50 mL of
water. Explain. Your answer must contain a well-thought-out and clearly written
explanation.
b) The buret is still wet with water on the inside when you add your NaOH solution.
Explain
c) The KHP is wet when you weigh it. Explain.
d) You titrate past the equivalence point by 0.50mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

