You are an engineer in a certain manufacturing plant. The plant manager suddenly gave you a task to procure an equipment that analyzes molecules. It was not your job, actually, but a job of a workmate yours (a friend of yours too). That workmate, an engineer too and was a previ- ous classmate of yours, had taken a leave and is currently taking a vacation in Morocco. Your workmate cannot be contacted as of the moment in any means (calls, SMS, and even in Messenger). The plant manager needs your report in a matter of 10 minutes. You decided to check the desk of your workmate and saw a data your workmate has been working: Material to analyze = water Change in entropy (J/K) = +300 Cp (J/g-K) = 4.183 T₁ (°C) = 25 T₂ (°C) = 80 Process = isobaric (constant pressure) After a minute or two of investigating, you suddenly knew what brand is to be procured. Determine the brand (see presented table). Equipment Brand Mustafa Jibril Zhang Wei Hoyota Kremshevik Norvark Kabayan Murica Suggested Capacity (Number of Molecules) 1.00×10¹4 to 2.00x10¹6 1.00×10¹6 to 2.00x10¹8 1.00x10¹8 to 2.00x102⁰ 1.00x1020 to 2.00x10²² 1.00×1022 to 2.00x10²4 1.00×1024 to 2.00x1026 1.00×1026 to 2.00x1028
You are an engineer in a certain manufacturing plant. The plant manager suddenly gave you a task to procure an equipment that analyzes molecules. It was not your job, actually, but a job of a workmate yours (a friend of yours too). That workmate, an engineer too and was a previ- ous classmate of yours, had taken a leave and is currently taking a vacation in Morocco. Your workmate cannot be contacted as of the moment in any means (calls, SMS, and even in Messenger). The plant manager needs your report in a matter of 10 minutes. You decided to check the desk of your workmate and saw a data your workmate has been working: Material to analyze = water Change in entropy (J/K) = +300 Cp (J/g-K) = 4.183 T₁ (°C) = 25 T₂ (°C) = 80 Process = isobaric (constant pressure) After a minute or two of investigating, you suddenly knew what brand is to be procured. Determine the brand (see presented table). Equipment Brand Mustafa Jibril Zhang Wei Hoyota Kremshevik Norvark Kabayan Murica Suggested Capacity (Number of Molecules) 1.00×10¹4 to 2.00x10¹6 1.00×10¹6 to 2.00x10¹8 1.00x10¹8 to 2.00x102⁰ 1.00x1020 to 2.00x10²² 1.00×1022 to 2.00x10²4 1.00×1024 to 2.00x1026 1.00×1026 to 2.00x1028
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
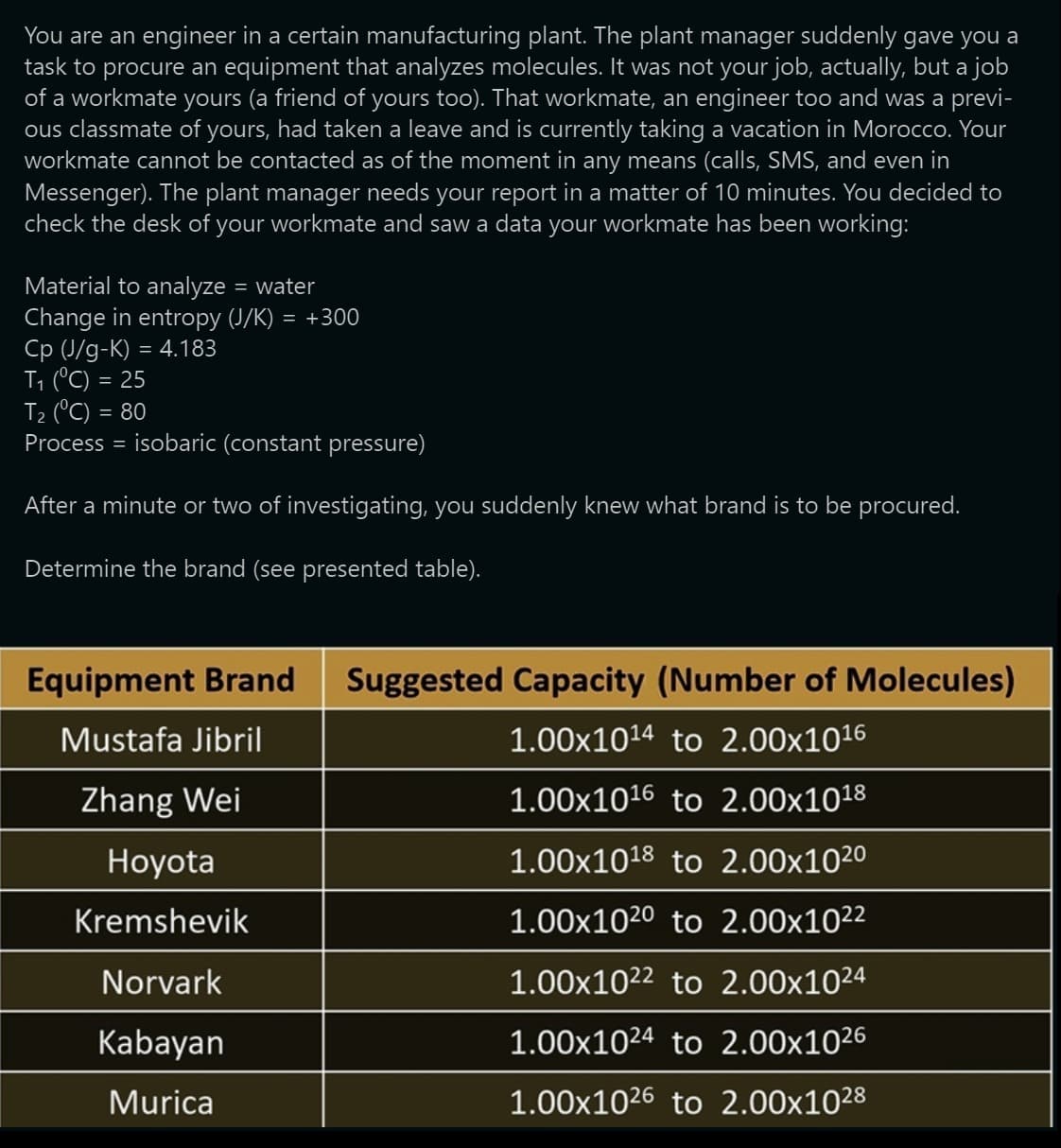
Transcribed Image Text:You are an engineer in a certain manufacturing plant. The plant manager suddenly gave you a
task to procure an equipment that analyzes molecules. It was not your job, actually, but a job
of a workmate yours (a friend of yours too). That workmate, an engineer too and was a previ-
ous classmate of yours, had taken a leave and is currently taking a vacation in Morocco. Your
workmate cannot be contacted as of the moment in any means (calls, SMS, and even in
Messenger). The plant manager needs your report in a matter of 10 minutes. You decided to
check the desk of your workmate and saw a data your workmate has been working:
Material to analyze = water
Change in entropy (J/K) = +300
Cp (J/g-K) = 4.183
T₁ (°C) = 25
T₂ (°C) = 80
Process = isobaric (constant pressure)
After a minute or two of investigating, you suddenly knew what brand is to be procured.
Determine the brand (see presented table).
Equipment Brand
Mustafa Jibril
Zhang Wei
Hoyota
Kremshevik
Norvark
Kabayan
Murica
Suggested Capacity (Number of Molecules)
1.00×10¹4 to 2.00x10¹6
1.00×10¹6 to 2.00x10¹8
1.00x10¹8 to 2.00x102⁰
1.00x1020 to 2.00x10²²
1.00×1022 to 2.00x1024
1.00x1024 to 2.00x10²6
1.00x1026 to 2.00x10²8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The