You are heating 310 mL of water to make tea; however, you forget the pot on the stove, and all of the water boils away. All of the steam quickly condenses onto the plastic shelf that hangs above the stove. The shelf is initially at 20°C, and heats uniformly to 33°C after all the steam condenses into water on it. The specific heat of water is 4190 J/kg K and that of plastic is 1670 J/kg K. The latent heat of fusion of water is 3.33x105 J/kg and the heat of vaporization of water is 22.6x105 J/kg. The density of water is 1000 kg/m³, and 1 mL = 10-6 m³. Your kitchen is at sea level. What is the mass of the shelf? Assume that no energy is lost to the environment.
You are heating 310 mL of water to make tea; however, you forget the pot on the stove, and all of the water boils away. All of the steam quickly condenses onto the plastic shelf that hangs above the stove. The shelf is initially at 20°C, and heats uniformly to 33°C after all the steam condenses into water on it. The specific heat of water is 4190 J/kg K and that of plastic is 1670 J/kg K. The latent heat of fusion of water is 3.33x105 J/kg and the heat of vaporization of water is 22.6x105 J/kg. The density of water is 1000 kg/m³, and 1 mL = 10-6 m³. Your kitchen is at sea level. What is the mass of the shelf? Assume that no energy is lost to the environment.
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 83P: To help prevent frost damage, 4.00 kg of water at 0 is sprayed onto a fruit tree. (a) How much heat...
Related questions
Question

Transcribed Image Text:You are heating 310 mL of water to make tea; however, you forget the pot on the
stove, and all of the water boils away. All of the steam quickly condenses onto the
plastic shelf that hangs above the stove. The shelf is initially at 20°C, and heats
uniformly to 33°C after all the steam condenses into water on it. The specific heat of
water is 4190 J/kg K and that of plastic is 1670 J/kg K. The latent heat of fusion of
water is 3.33×105 J/kg and the heat of vaporization of water is 22.6x105 J/kg. The
density of water is 1000 kg/m3, and 1 mL = 10-6 m³. Your kitchen is at sea level.
What is the mass of the shelf? Assume that no energy is lost to the environment.
Expert Solution
Step 1
The specific heat (C) and latent heat (L) of a substance of mass (m) that absorbs heat (Q) and its temperature changes by (ΔT) or absorbs heat (Q’) and its phase changes may be expressed as follows:
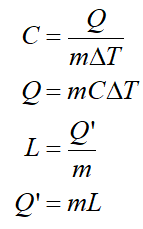
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning