Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
#2 pls help me
Transcribed Image Text:EEL 20 X
A 30 YerX
I E&EL X
U.S.I X
TE&EL X
US: X
+EGEL X
O us Inf x
A app.teachermade.com/fill/e5b84085-60dd-40a5-9253-aeb1d96b5eff
Middletown Bookmarks
FreeCollegeSchedul.
FreeCollegeSchedul.
FreeCollegeSchedul..
FreeCollegeSchedul..
T
T 14
ve for Later
I'm Done
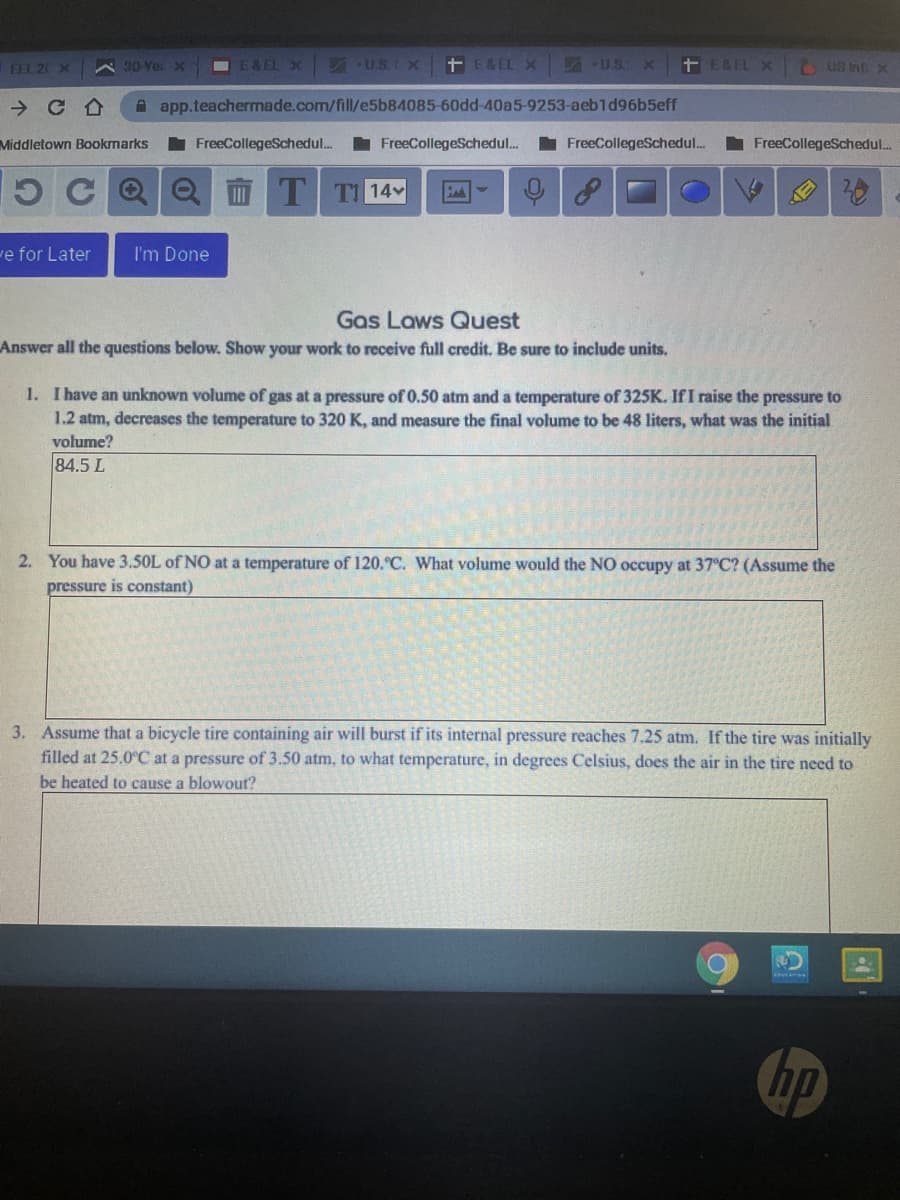
Gas Laws Quest
Answer all the questions below. Show your work to receive full credit. Be sure to include units.
1. I have an unknown volume of gas at a pressure of 0.50 atm and a temperature of 325K. If I raise the pressure to
1.2 atm, decreases the temperature to 320 K, and measure the final volume to be 48 liters, what was the initial
volume?
84.5 L
2. You have 3.50L of NO at a temperature of 120.°C. What volume would the NO occupy at 37°C? (Assume the
pressure is constant)
3. Assume that a bicycle tire containing air will burst if its internal pressure reaches 7.25 atm. If the tire was initially
filled at 25.0°C at a pressure of 3.50 atm, to what temperature, in degrees Celsius, does the air in the tire need to
be heated to cause a blowout?
ap
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

