You wish to make compound D. You have the starting materials E, F, or G and a range of reagent(s) available to you, as given below. The shortest route is three steps. Outline how you would achieve maximum yield of product D. In your answer clearly note the starting material chosen, the structure of the intermediates and note the reagents you would use to perform each step. HO CH3 'Retrosynthesis arrow' 'Retrosynthesis arrow' 'Retrosynthesis arrow' CH3 ОН step 3 step 2 step 1 D intermediate II intermediate l Starting material E, F or G Starting material (only choose one) HO CH3 CH3 CH3 `CH3 `CH3 `CH3 HO, E F G Reagents and solvents available to you РСС, Н2О Dess-Martin Periodinane, CH2C12 Na2Cr207, H2SO4, acetone LIAIH4, THF NaBHa, MeOН На, Pа/C, MeOН (CH3)3SICI, imidazole, CH2C12 (CH3)2('Bu)SiCl, imidazole, CH2C12 BnBr, NaH, THF Bu4N+F» THF H*/H2O
You wish to make compound D. You have the starting materials E, F, or G and a range of reagent(s) available to you, as given below. The shortest route is three steps. Outline how you would achieve maximum yield of product D. In your answer clearly note the starting material chosen, the structure of the intermediates and note the reagents you would use to perform each step. HO CH3 'Retrosynthesis arrow' 'Retrosynthesis arrow' 'Retrosynthesis arrow' CH3 ОН step 3 step 2 step 1 D intermediate II intermediate l Starting material E, F or G Starting material (only choose one) HO CH3 CH3 CH3 `CH3 `CH3 `CH3 HO, E F G Reagents and solvents available to you РСС, Н2О Dess-Martin Periodinane, CH2C12 Na2Cr207, H2SO4, acetone LIAIH4, THF NaBHa, MeOН На, Pа/C, MeOН (CH3)3SICI, imidazole, CH2C12 (CH3)2('Bu)SiCl, imidazole, CH2C12 BnBr, NaH, THF Bu4N+F» THF H*/H2O
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.36P
Related questions
Question
Need help with this question. Thank you :)
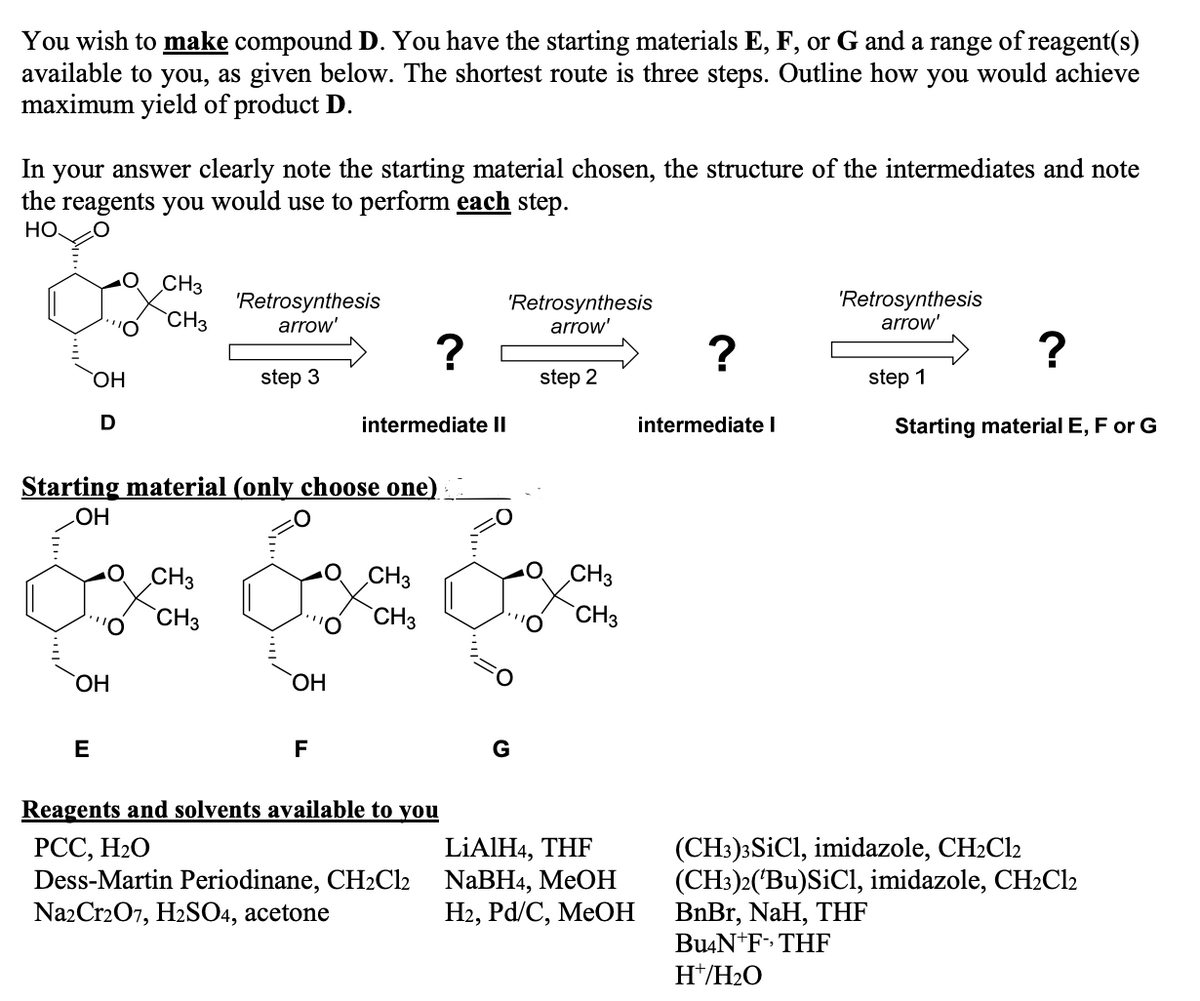
Transcribed Image Text:You wish to make compound D. You have the starting materials E, F, or G and a range of reagent(s)
available to you, as given below. The shortest route is three steps. Outline how you would achieve
maximum yield of product D.
In your answer clearly note the starting material chosen, the structure of the intermediates and note
the reagents you would use to perform each step.
НО
CH3
'Retrosynthesis
arrow'
'Retrosynthesis
arrow'
'Retrosynthesis
arrow'
CH3
?
HO,
step 3
step 2
step 1
D
intermediate II
intermediate I
Starting material E, F or G
Starting material (only choose one)
HO
CH3
O CH3
CH3
CH3
CH3
`CH3
HO,
ОН
E
F
Reagents and solvents available to you
РСС, Н2О
Dess-Martin Periodinane, CH2Cl2
NazCr207, H2SO4, acetone
LİAIH4, THF
NaBH4, MEOH
На, Pd/C, MeOН
(CH3)3SICI, imidazole, CH2C12
(CH:)2('Bu)SiCl, imidazole, CH2C12
BnBr, NaH, THF
Bu4N+F» THF
H*/H2O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning