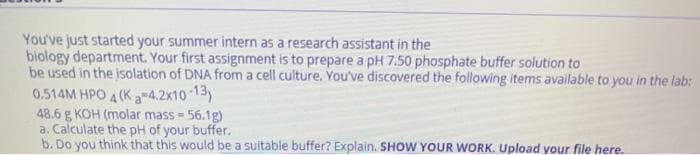
You've just started your summer intern as a research assistant in the biology department. Your first assignment is to prepare a pH 7.50 phosphate buffer solution to be used in the isolation of DNA from a cell culture. You've discovered the following items available to you in the lab: 0.514M HPO 4 (K a-4.2x10 13) 48.6 g KOH (molar mass - 56.1g) a. Calculate the pH of your buffer. b. Do you think that this would be a suitable buffer? Explain. SHOW YOUR WORK. Upload your file here.
You've just started your summer intern as a research assistant in the biology department. Your first assignment is to prepare a pH 7.50 phosphate buffer solution to be used in the isolation of DNA from a cell culture. You've discovered the following items available to you in the lab: 0.514M HPO 4 (K a-4.2x10 13) 48.6 g KOH (molar mass - 56.1g) a. Calculate the pH of your buffer. b. Do you think that this would be a suitable buffer? Explain. SHOW YOUR WORK. Upload your file here.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 106GQ: The hydrogen phthalate ion, C8HsO4, is a weak acid with Ka = 3.91 106....
Related questions
Question
Transcribed Image Text:You've just started your summer intern as a research assistant in the
biology department. Your first assignment is to prepare a pH 7.50 phosphate buffer solution to
be used in the isolation of DNA from a cell culture. You've discovered the following items available to you in the lab:
0.514M HPO 4 (K a-4.2x10 13)
48.6 g KOH (molar mass 56.1g)
a. Calculate the pH of your buffer.
b. Do you think that this would be a suitable buffer? Explain. SHOW YOUR WORK. Upload your file here.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning