Zinc and sodium metal each react with sulfuric acid according to the following equations. Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g)Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g) 2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g)2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g) A 26.14 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then reacted with 264 mL of 1.86 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the percent sodium by mass in the original mixture. 1.)What is the mass percent Na?
Zinc and sodium metal each react with sulfuric acid according to the following equations. Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g)Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g) 2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g)2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g) A 26.14 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then reacted with 264 mL of 1.86 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the percent sodium by mass in the original mixture. 1.)What is the mass percent Na?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.111PAE: 4.111 Aluminum metal reacts with sulfuric acid to form hydrogen gas and aluminum sulfate (a) Write a...
Related questions
Question
Zinc and sodium metal each react with sulfuric acid according to the following equations.
Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g)Zn(s)+H2SO4(aq)⟶ZnSO4(aq)+H2(g)
2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g)2Na(s)+H2SO4(aq)⟶Na2SO4(aq)+H2(g)
A 26.14 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then reacted with 264 mL of 1.86 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the percent sodium by mass in the original mixture.
1.)What is the mass percent Na?

Transcribed Image Text:Na, SO,(aq) + BaCl, (aq) → 2 NaCl(aq) + BaSO,(s)
Since the maximum quantity of barium sulfate was produced, all of the BaCl,
was converted into BaSO, and the moles of BaCl, is equal to the moles of
BaSO,. Use the molarity and volume to find the moles of barium chloride.
4*
The barium sulfate produced came from both zinc sulfate and sodium sulfate
and each was produced in a 1:1 mole ratio. Therefore,
mol BaSO, = mol ZnSO, + mol Na, SO,
Since the question asks for the mass of sodium metal in the mixture, relate the
amounts of zinc sulfate and sodium sulfate to zinc metal and sodium metal.
From the balanced chemical equations for the reaction of these metals with
sulfuric acid, zinc produces zinc sulfate is a 1:1 mole ratio and sodium
produces sodium sulfate in a 2:1 mole ratio. Therefore,
1
= mol Zn + =mol Na
2
mol BaSO4
Express the moles of zinc and sodium in terms of the masses of each (x and y)
and the molar masses of each. Then, write a second equation relating x and y
to the mass of the mixture.
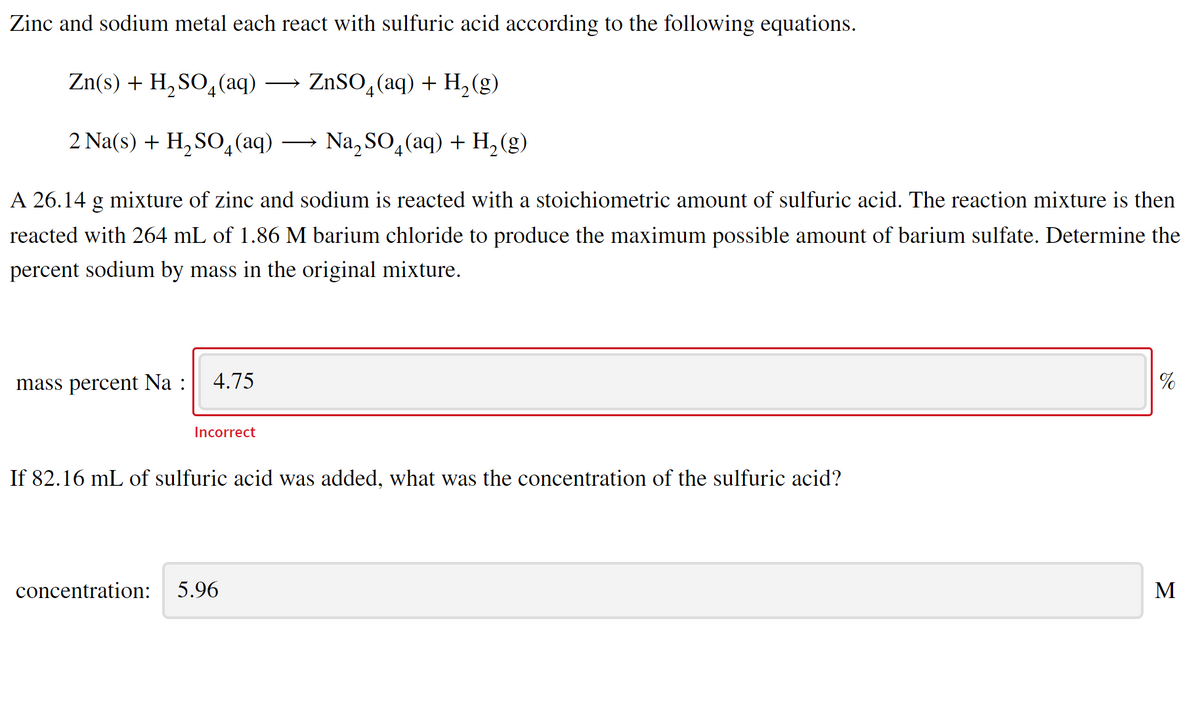
Transcribed Image Text:Zinc and sodium metal each react with sulfuric acid according to the following equations.
Zn(s) + H, SO,(aq) → ZnSO,(aq) + H, (g)
2.
4
2 Na(s) + H, SO4(aq) → Na, SO4(aq) + H, (g)
A 26.14 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. The reaction mixture is then
reacted with 264 mL of 1.86 M barium chloride to produce the maximum possible amount of barium sulfate. Determine the
percent sodium by mass in the original mixture.
mass percent Na :
4.75
%
Incorrect
If 82.16 mL of sulfuric acid was added, what was the concentration of the sulfuric acid?
concentration:
5.96
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning