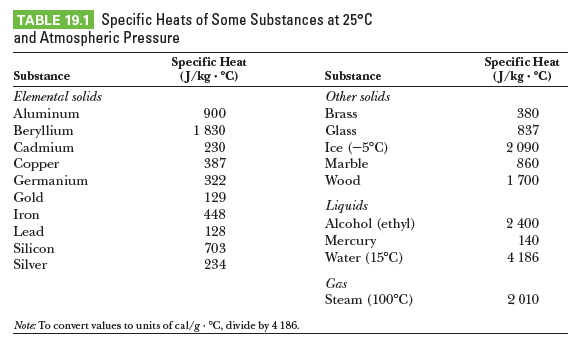
TABLE 19.1 Specific Heats of Some Substances at 25°C and Atmospheric Pressure Specific Heat J/kg - °C) Specific Heat (J/kg . °C) Substance Substance Elemental solids Other solids 900 1 830 Aluminum Brass 380 Beryllium Glass 837 Cadmium 230 387 Ice (-5°C) 2 090 Copper Marble 860 Germanium 322 Wood 1 700 Gold 129 Liquids Alcohol (ethyl) Mercury Water (15°C) Iron 448 2 400 Lead 128 703 140 Silicon 4 186 Silver 234 Gas Steam (100°C) 2 010 Note: To convert values to units of cal/g °C, divide by 4 186.
Energy transfer
The flow of energy from one region to another region is referred to as energy transfer. Since energy is quantitative; it must be transferred to a body or a material to work or to heat the system.
Molar Specific Heat
Heat capacity is the amount of heat energy absorbed or released by a chemical substance per the change in temperature of that substance. The change in heat is also called enthalpy. The SI unit of heat capacity is Joules per Kelvin, which is (J K-1)
Thermal Properties of Matter
Thermal energy is described as one of the form of heat energy which flows from one body of higher temperature to the other with the lower temperature when these two bodies are placed in contact to each other. Heat is described as the form of energy which is transferred between the two systems or in between the systems and their surrounding by the virtue of difference in temperature. Calorimetry is that branch of science which helps in measuring the changes which are taking place in the heat energy of a given body.
A student measures the following data in a calorimetry experiment designed to determine the specific heat of aluminum:
Initial temperature of water and calorimeter: 70.08C
Mass of water: 0.400 kg
Mass of calorimeter: 0.040 kg
Specific heat of calorimeter: 0.63 kJ/kg ? °C
Initial temperature of aluminum: 27.0°C
Mass of aluminum: 0.200 kg
Final temperature of mixture: 66.3°C
(a) Use these data to determine the specific heat of aluminum.
(b) Explain whether your result is within 15% of the value listed as shown.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images









