Putrescine, a substance produced by decaying animals, has the empirical formula C2H6N. Several determinations of molecular weight give values in the range of 87 to 90 amu. Find the molecular formula of putrescine.
Putrescine, a substance produced by decaying animals, has the empirical formula C2H6N. Several determinations of molecular weight give values in the range of 87 to 90 amu. Find the molecular formula of putrescine.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 128CP: itamin B12 , cyancobalamin, is essential for human nutrition. Its molecular formula is...
Related questions
Question
Putrescine, a substance produced by decaying animals, has the empirical formula C2H6N. Several determinations of molecular weight give values in the range of 87 to 90 amu. Find the molecular formula of putrescine.
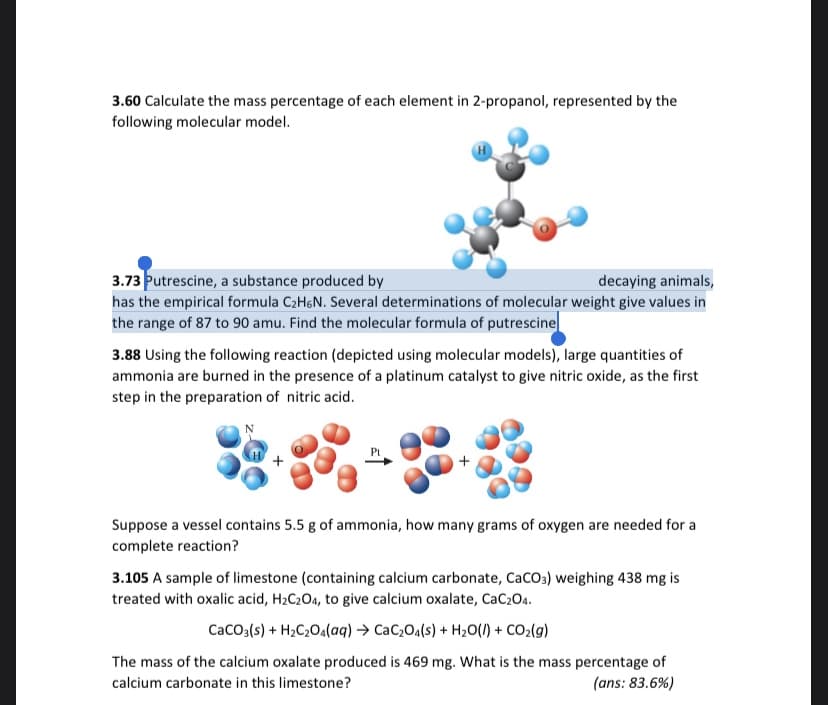
Transcribed Image Text:3.60 Calculate the mass percentage of each element in 2-propanol, represented by the
following molecular model.
3.73 Putrescine, a substance produced by
has the empirical formula C2H6N. Several determinations of molecular weight give values in
the range of 87 to 90 amu. Find the molecular formula of putrescine
decaying animals,
3.88 Using the following reaction (depicted using molecular models), large quantities of
ammonia are burned in the presence of a platinum catalyst to give nitric oxide, as the first
step in the preparation of nitric acid.
Suppose a vessel contains 5.5 g of ammonia, how many grams of oxygen are needed for a
complete reaction?
3.105 A sample of limestone (containing calcium carbonate, CaCO3) weighing 438 mg is
treated with oxalic acid, H2C204, to give calcium oxalate, CaC2O4.
CaCO3(s) + H2C20a(aq) → CaC2Oa(s) + H20(1) + CO2(g)
The mass of the calcium oxalate produced is 469 mg. What is the mass percentage of
(ans: 83.6%)
calcium carbonate in this limestone?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning