How Many Neutrons Does a Hydrogen Atom Have?
Answer – The number of neutrons in a hydrogen atom depends on the specific isotope of hydrogen, and the most common isotope, protium, has no neutrons.
Explanation:
Neutrons are subatomic particles found in the nucleus of an atom with protons. They are electrically neutral. The number of neutrons in an atom can vary, even for isotopes.
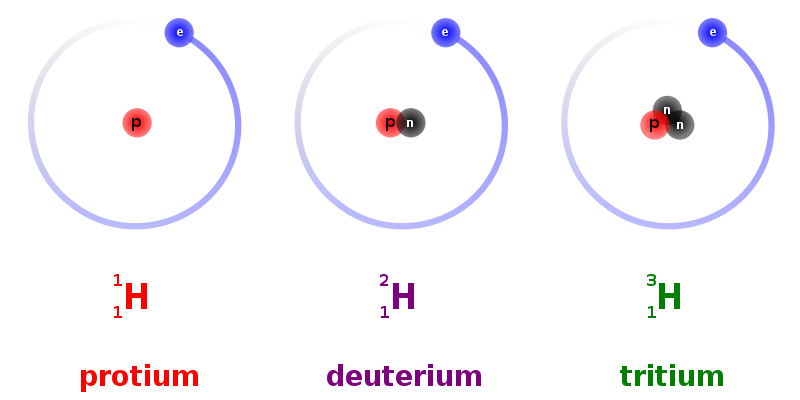
Image credit: Dirk Hünniger / Wikimedia Commons (licensed under CC BY-SA 3.0)
The hydrogen atom has three isotopes: protium, deuterium, and tritium. The most common one, protium, has one proton and zero neutrons in its nucleus. Deuterium (²H) has one neutron, whereas tritium (³H) has two neutrons.