. A general expression for the energy levels of one-electron atoms and ions is uk q°q² E, 2h'n? Here u is the reduced mass of the atom, given by u = m, m,/ (m, + m2), where m is the mass of the electron and m, is the mass of the nucleus; k, is the Coulomb constant; and q and 2 are the charges of the electron and the nucleus, respec- tively. The wavelength for the n= 3 to n = 2 transition of the hydrogen atom is 656.3 nm (visible red light). What are the wavelengths for this same transition in (a) positronium, which consists of an electron and a positron, and (b) singly ionized helium? Note: A positron is a positively charged electron.
. A general expression for the energy levels of one-electron atoms and ions is uk q°q² E, 2h'n? Here u is the reduced mass of the atom, given by u = m, m,/ (m, + m2), where m is the mass of the electron and m, is the mass of the nucleus; k, is the Coulomb constant; and q and 2 are the charges of the electron and the nucleus, respec- tively. The wavelength for the n= 3 to n = 2 transition of the hydrogen atom is 656.3 nm (visible red light). What are the wavelengths for this same transition in (a) positronium, which consists of an electron and a positron, and (b) singly ionized helium? Note: A positron is a positively charged electron.
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter28: Atomic Physics
Section: Chapter Questions
Problem 29P
Related questions
Question
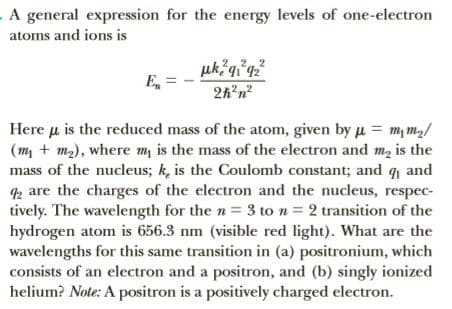
Transcribed Image Text:. A general expression for the energy levels of one-electron
atoms and ions is
uk q°q²
E,
2h'n?
Here u is the reduced mass of the atom, given by u = m, m,/
(m, + m2), where m is the mass of the electron and m, is the
mass of the nucleus; k, is the Coulomb constant; and q and
2 are the charges of the electron and the nucleus, respec-
tively. The wavelength for the n= 3 to n = 2 transition of the
hydrogen atom is 656.3 nm (visible red light). What are the
wavelengths for this same transition in (a) positronium, which
consists of an electron and a positron, and (b) singly ionized
helium? Note: A positron is a positively charged electron.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning
