. A linear plot of molar conductivity versus square root of concentration for AB2 electrolyte gives an intercept of 0.01974 S m²/mol and slope of 0.500. Whereas a plot of reciprocal molar conductivity versus concentration-multiplied by-molar conductivity of acid HB give an intercept of 25.27 mol/S m² (at 25 °C, Ka of HB = 1.80 x 10*). What is the ionic equivalent conductance value for A²+ ion? (considering that H* = 0.0350 S m/mol)
. A linear plot of molar conductivity versus square root of concentration for AB2 electrolyte gives an intercept of 0.01974 S m²/mol and slope of 0.500. Whereas a plot of reciprocal molar conductivity versus concentration-multiplied by-molar conductivity of acid HB give an intercept of 25.27 mol/S m² (at 25 °C, Ka of HB = 1.80 x 10*). What is the ionic equivalent conductance value for A²+ ion? (considering that H* = 0.0350 S m/mol)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 52PS: The dispersed phase of a certain colloidal dispersion consists of spheres of diameter 1.0 102 nm....
Related questions
Question
help.
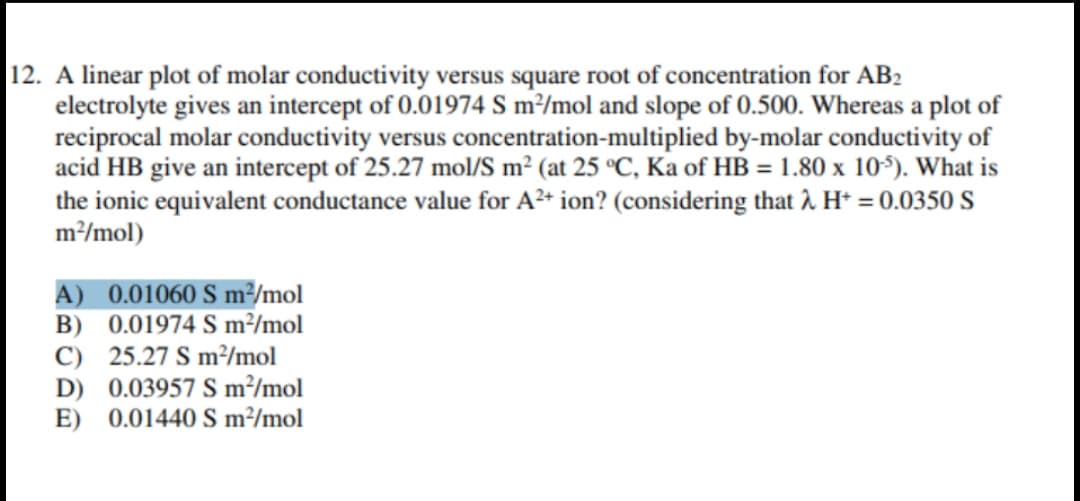
Transcribed Image Text:12. A linear plot of molar conductivity versus square root of concentration for AB2
electrolyte gives an intercept of 0.01974 S m²/mol and slope of 0.500. Whereas a plot of
reciprocal molar conductivity versus concentration-multiplied by-molar conductivity of
acid HB give an intercept of 25.27 mol/S m² (at 25 °C, Ka of HB = 1.80 x 10). What is
the ionic equivalent conductance value for A²* ion? (considering that î. H* = 0.0350 S
m/mol)
A) 0.01060 S m²/mol
B) 0.01974 S m²/mol
C) 25.27 S m²/mol
D) 0.03957 S m²/mol
E) 0.01440 S m²/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning