. The freezing point depression of a solution of glucose (C,H,206, molar mass = 180.0 g/mol) is -10.3°C. Calculate for the molarity of the glucose solution given that the density of the solution is 1.50 g/mL and the freezing point constant, K¢, of water is 1.86 °C kg mol·1.
. The freezing point depression of a solution of glucose (C,H,206, molar mass = 180.0 g/mol) is -10.3°C. Calculate for the molarity of the glucose solution given that the density of the solution is 1.50 g/mL and the freezing point constant, K¢, of water is 1.86 °C kg mol·1.
An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter13: Chemical Reactions
Section: Chapter Questions
Problem 38SA: Suppose you are given the volume (in liters) of a salt (NaCl) solution and its molarity. Explain how...
Related questions
Question
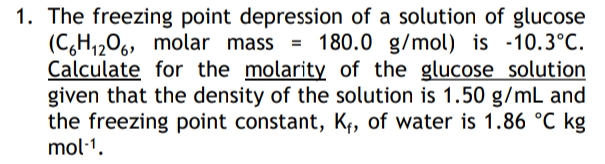
Transcribed Image Text:1. The freezing point depression of a solution of glucose
(C,H1206, molar mass
Calculate for the molarity of the glucose solution
given that the density of the solution is 1.50 g/mL and
the freezing point constant, K¢, of water is 1.86 °C kg
mol·1.
= 180.0 g/mol) is -10.3°C.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning