. Which of the following statements is true regarding the periodic table? a. elements of the same group have the same oxidation number b. elements are arranged based on decreasing atomic weight c. both are correct d. both are incorrect 2. Please refer to the picture. The molecular geometry of the right-most carbon in the molecule is: a. trigonal bipyramidal b. trigonal planar c. pyramidal d. tetrahedral 3. Given the following statements: I. The less electronegative atom gains a greater fraction of the shared electron thereby acquiring a partial positive charge indicated by delta plus. II. Increasing the differences in electronegativity is indicative of decreasing bond polarity. a. both are correct b. both are incorrect c. statement 1 is correct d. statement 2 is correct e. statement 1 is incorrect f. statement 2 is incorrect
1. Which of the following statements is true regarding the periodic table?
a. elements of the same group have the same oxidation number
b. elements are arranged based on decreasing atomic weight
c. both are correct
d. both are incorrect
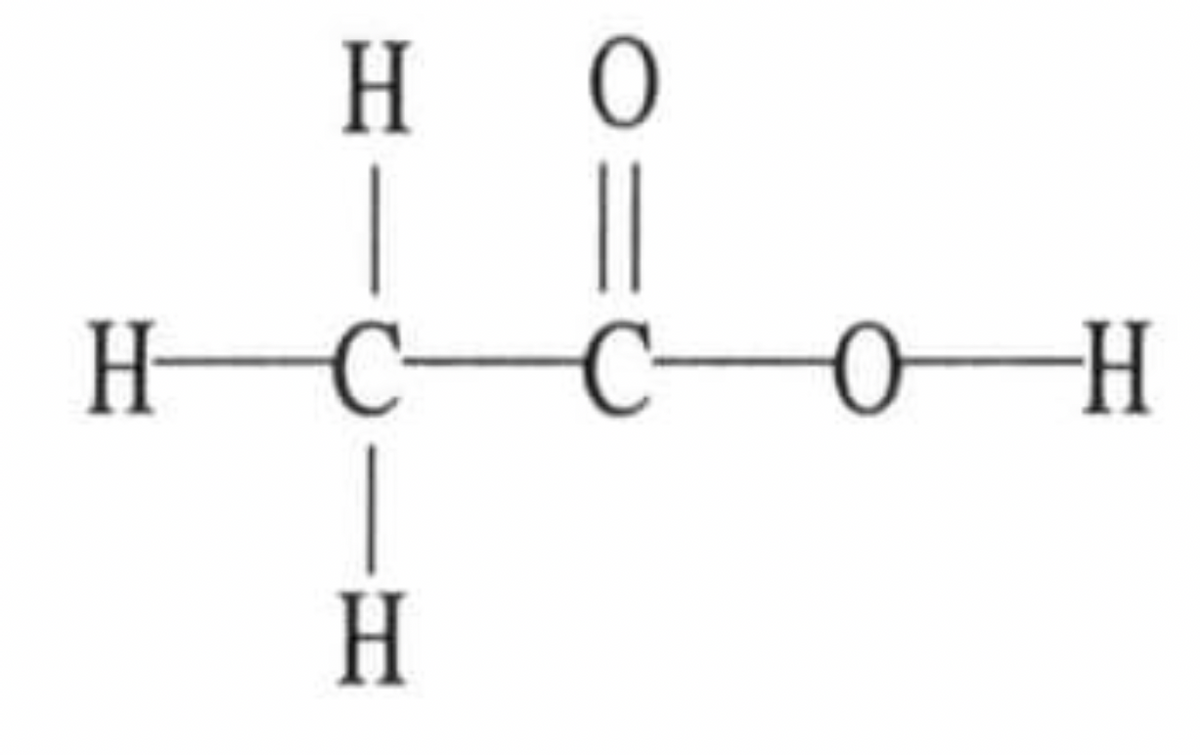
2. Please refer to the picture. The molecular geometry of the right-most carbon in the molecule is:
a. trigonal bipyramidal
b. trigonal planar
c. pyramidal
d. tetrahedral
3. Given the following statements:
I. The less electronegative atom gains a greater fraction of the shared electron thereby acquiring a partial positive charge indicated by delta plus.
II. Increasing the differences in electronegativity is indicative of decreasing bond polarity.
a. both are correct
b. both are incorrect
c. statement 1 is correct
d. statement 2 is correct
e. statement 1 is incorrect
f. statement 2 is incorrect
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images









