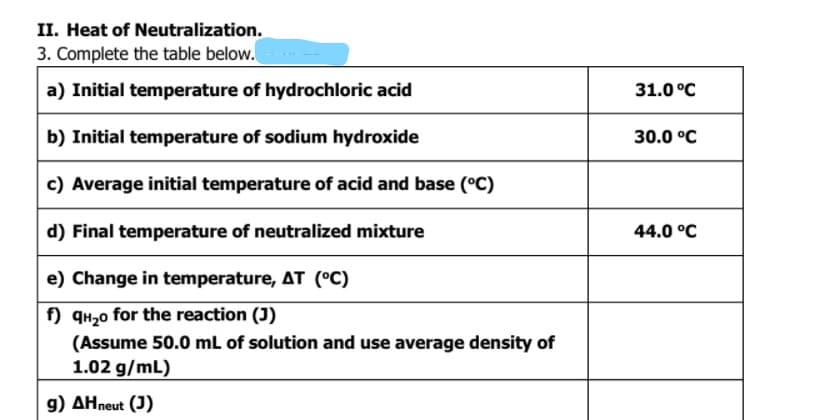
.Heat of Neutralization.
Q: Which of the following is a genetic disorder resulting in defective assembly of a chloride transport…
A: A genetic disorder is a disease that is caused by a change, or mutation, in an individual's DNA…
Q: 1. Draw a standard curve of absorbance at 420nm against glucose concentration in mmoles/L. We…
A: Glucose concentration of an unknown sample can be calculated by using a standard curve, which is…
Q: What is measured at the A260 absorbance values?
A: DNA and RNA absorbs UV light due to heterocyclic rings of the nucleotides that is present in the…
Q: Briefly describe the middle of the Ion exchanger Chromatography
A:
Q: Example of protective proteins
A: A defensive protein is delivered by the resistant framework in light of the presence of a foreign…
Q: areas of high solute concentration to areas of low solute concentration
A: The correct answer is B . Areas of high solute concentration to area of low solute concentration
Q: Principle underlying neutralization tests.
A: Antibodies are specific proteins that are secreted by the body to kill or deactivate the harmful…
Q: rapy and several topical treatments in the past but they eventually became ineffective. He uses…
A: It is auto immune disorder of skin. Characterized by inflammatory erythematous and papulosquamous…
Q: Observations: Viscosity of the Syrup
A: Thick fluids like maple syrup, corn syrup, and nectar are said to have a tall "thickness," where the…
Q: MICROELEMENT COMING INTO THE BODY WITH WATER 1. iodine 2. bromine 3. sodium 4. Fluorine 5. silver
A: Water hydrates mucosal membranes in the respiratory and gastrointestinal systems and is required for…
Q: Thermal Injury: Scalding Why is it important to document the distribution of the burn injury in…
A: Scalding is a thermal injury to the skin and tissues . It is caused by the hot liquid. Scalding…
Q: Define the electrochemical gradient and describe its components.
A: Lipid bilayer is intrinsically impermeable to ions to polar molecules, yet certain molecules must be…
Q: What Type of guard column, separation column, and suppressor used for anion- exchange chromatography
A: In the anion-exchange chromatography, the process of separation occurs which is based on the charges…
Q: Viscosity and density fatty acid solution at room temparature
A: Viscosity, density : both decrease with the temperature increase because a larger temperature means…
Q: Define contact time in adsorption
A: Adsorption - Process by which a substance such as solid, takes up another substance such as liquid…
Q: 5, Regarding chemical mediators
A: Inflammation: It is a non specific defense reaction to the cell injury. It causes pain, redness,…
Q: Define critical concentration,
A: To define: To define critical concentration and its functions involved in it
Q: 1. 2. 3. A* 4. H-
A: The solvation of a solute by water is called hydration.
Q: When doing the glucose in drinks experiment I measured an absorbance reading of 0.002 for orange…
A: Light is made up of many colors, which are referred to as wavelengths . Each color has its own…
Q: Does increasing or decreasing the path length affect absorbance? Explain.
A: Introduction: Photometry frames a significant research facility device for precise assessment of a…
Q: Determine the amount of ascorbic acid in the unknown sample using the standard concentrations and…
A: Given : Absorbance at different concentrations Unknown concentration(C) = (Test absorbance/Standard…
Q: Briefly describe the difference between chromatography and ion exchange
A: Asked : Difference between chromatography and ion exchange
Q: What is the function of mordant?
A: Gram staining is a common technique that is used to differentiate bacteria into two broad groups…
Q: extracellular conditions are unfavorable, th
A:
Q: Explain why a broad-spectrum sunscreen would contain both molecules: 2-EHMC and TDSA.
A: The sunscreen is a substance that protects the skin from the harmful rays of the sunlight resulting…
Q: What is the relevance of measuring absorbance against protein concentration?
A: Introduction : Proteins are nitrogenous organic substances made up of one or more elongated chains…
Q: Is there are direct relationship between the values of percent transmittance and absorbance? Provide…
A: Transmittance and absorbance are well known terms in the field of spectroscopy. Spectroscopy…
Q: molecule in each ionization state. H|| H,N*-C-C- OH H|| H,N-C-C-0- H I H,N-C-C-0- H,N-C-C-C CH, CH,…
A: pH is the measure of the strength of H+ ion or Hydronium ions in solution. pOH is the…
Q: Graph of Absorbance vs Time(mins) 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 10 20 30 40 50 60 70 80 Time…
A: Growth rate of bacteria is the speed or rate at which the bacterial population grows per unit time…
Q: Equation involved in Molisch test
A: Molisch's test is the qualitative test that is used to detect the presence of carbohydrates such as…
Q: Hypotonic solutions cause red blood cells to shrivel- a process known as crenation.
A: Osmosis is the diffusion of water molecules across a selectively permeable membrane from a region of…
Q: The inorganic compound most abundant on plasma cell is
A: - Plasma cells are differentiated B-lymphocyte white blood cells . they're capable of secreting…
Q: Principle of Neutralization Tests (NT).
A: In a neutralization test, serum and virus are reacted together in equal volumes and inoculated into…
Q: Membrane in Column Chromatography In column chromatography, the is the membrane that allows water to…
A: Chromatography is a laboratory technique for separating a mixture in chemical analysis. The…
Q: Clare Boothe Luce, ambassador to Italy in the 1950s (and Connecticut congressperson, playwright,…
A: Introduction: Cupric arsenite is a carcinogen known to cause cancer in humans along with other…
Q: why should the ethanol/lystate boundary not be distrubed
A: Ethanol lysate boundary is very much important in the case of DNA extraction experiments, where…
Q: A paramecium cell placed into a hypotonic solution will experience which of the following effects?…
A: Osmosis is a process in which water molecules from the low concentration of solute to the areas…
Q: Which of the following classes of compounds are biological surfactants and one of the main…
A: Biological Surfactants, also known as Biosurfactants are the surfactants that are derived from…
Q: Briefly explain why alpha-linolenic acid is essential
A: - alpha-linolenic acid is essential because of the following factors: They help in reducing risks…
Q: Give a precise definition of the porphyrin ring, explain how it relates to ions and molecules, and…
A: Porphyrins are a class of heterocyclic macrocycle organic compounds made up of four modified pyrrole…
Q: Light Intensity 20 Temperature 25 Light Color = White Explain what happened to the dissolved…
A: Light energy is used by plants in order to Carbon into useful compounds like glucose, the breakdown…
Q: Many heavy metals are used in antiseptics. Explain the oligodynamic effect and provide a clinical…
A: Heavy metals can be defined as those chemical elements that have a relatively higher density than…
Q: Phosphorus immobilization occurs when:
A: Immobilization of minerals Immobilization is reverse process of mineralisation. In immobilization,…
Q: Suggest a reason why inorganic ions, such K+, Na+, Ca2+ and Mg2+, do not cross biological membranes…
A: Biological membranes separate the inside and outside of an organism and controls the entry and exit…
Q: Water could moves across plasma member through simple diffusion or facilitated diffusion, energy is…
A: Owing to the high osmotic pressure difference between the inside and the outside of the cell, water…
Q: Explain how soap works, include a discussion of the polarity, solubility of both the grease and…
A: The structure of soap includes a long hydrocarbon chain at one end and an iconic and at other end.…
Q: Glycophospholipid that is associated with lung surfactant
A: Lung surfactant is a mixture of lipids and proteins which is secreted into the alveolar space by…
Q: The function of centrioles includes
A: Centrioles are organelle present in animal cells. They are barrel shaped and are made up of tubulin…
Q: n human exist in chemically defined media for 48 hours?
A: A chemically defined medium is one whose constituents and concentrations (or quantities) are known.…
Step by step
Solved in 2 steps with 2 images

- Adult patient, admitted to a hospital, receives prescription of 25% zinc oxide ointment. Whereas the pharmacy only provided 480 grams of zinc oxide ointment at 10% concentration, how many grams of zinc oxide should be incorporated to prepare the prescribed ointment?Why is picric acid used for burns? Explain the principle involvedIf a powder in a 2gm vial diluted with 6.8ml of diluent provides 8ml of solution and each ml contains 250mg of a drug, how many ml is needed to give 750mg.
- NEORAL oral solution contains 100 mg/mL of cyclosporine. If a pharmacist prepares 30 mL of an oral solution containing 10% w/v cyclosporine, how many milliliters of diluent should be used?1. What volume of sterile water for injection (diluent) must be added to reconstitue the powder? 2. How many mL of the reconstituted cefazolin is needed to prepare the final product?Which organic molecules may be found in urine from a patient with kidney illness, and which biochemical test would be the most suited for detecting those molecues?
- Prepare a complete analysis procedure using KMnO4 as teh oxidant instead of K2CrO7 in the redox analysis of iron. Include sample and solution preparation, approximate weights of samples and reagents, procedure and chemical reactions. In particular, what is the purpose of the Zimmermann-Reinhardt reagent?(I) Why the physicochemical changes in liquid water caused by radiation is the key to understanding the biological effects of radiation? Please give a short, one-sentence explanation. (II) Name the three key stages of the physicochemical changes produced in liquid water due to radiation. (III) Briefly describe the three key stages. A one- or two-sentence explanation for each of the three key stages would be sufficient.based on safety information for the use of Ethidium Bromide solution and SybrSafe™. What makes SybrSafe™ safer than Ethidium Bromide?





