0 Properties computed with: NIST REFPROP version 7.0 100 ENTHALPY, kJ/kg 200 300 400 5 0.01 0.02 + 0.40- 0.50 0.60 -0.70 0.80 0.90 1.00 1.10. 1.20 1.30 1.40 1.50 1.60 1.70 1.80 1.90 2.00 2.10 2.20 2.30 2.40 2.50 0.04 0.06 0.08 0.1 Copy Saturated Liquid 0.1 0.2. 0.3- x=0.4 0.5 0.6: 0.7 0.8 0.93 Saturated Vap PRESSURE, MPa 0.2 -10 2 -80 -70 -60 -50°C -40 -30 -20 4 1300 1250: 1200 1150- 8-8 8... 8. 6 8 -10 0 > 10 20 30- 40 :50 Reference state: for saturated liquid at 0°C h = 200.0 kJ/kg, s= 1.00 kJ/(kg- -09 70 8.12. 10 ....... (difluoromethane) R-32 Ex 1100 1050- 1000 006 800 700, 009 -500- X 20 0 100 200 300 400
0 Properties computed with: NIST REFPROP version 7.0 100 ENTHALPY, kJ/kg 200 300 400 5 0.01 0.02 + 0.40- 0.50 0.60 -0.70 0.80 0.90 1.00 1.10. 1.20 1.30 1.40 1.50 1.60 1.70 1.80 1.90 2.00 2.10 2.20 2.30 2.40 2.50 0.04 0.06 0.08 0.1 Copy Saturated Liquid 0.1 0.2. 0.3- x=0.4 0.5 0.6: 0.7 0.8 0.93 Saturated Vap PRESSURE, MPa 0.2 -10 2 -80 -70 -60 -50°C -40 -30 -20 4 1300 1250: 1200 1150- 8-8 8... 8. 6 8 -10 0 > 10 20 30- 40 :50 Reference state: for saturated liquid at 0°C h = 200.0 kJ/kg, s= 1.00 kJ/(kg- -09 70 8.12. 10 ....... (difluoromethane) R-32 Ex 1100 1050- 1000 006 800 700, 009 -500- X 20 0 100 200 300 400
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter46: Room Air Conditioners
Section: Chapter Questions
Problem 15RQ
Related questions
Question
A Single Stage Vapor Compression (SSVC) refrigeration system has a cooling capacity of 7.5 tons. The following are the actual conditions: discharge of the evaporator is at -30°C & 0.2 MPa, discharge of the condenser is at 30°C & 2.0 MPa, discharge of the compressor is at 130°C & 2.5 MPa. Determine the Refrigerating Efficiency of the system
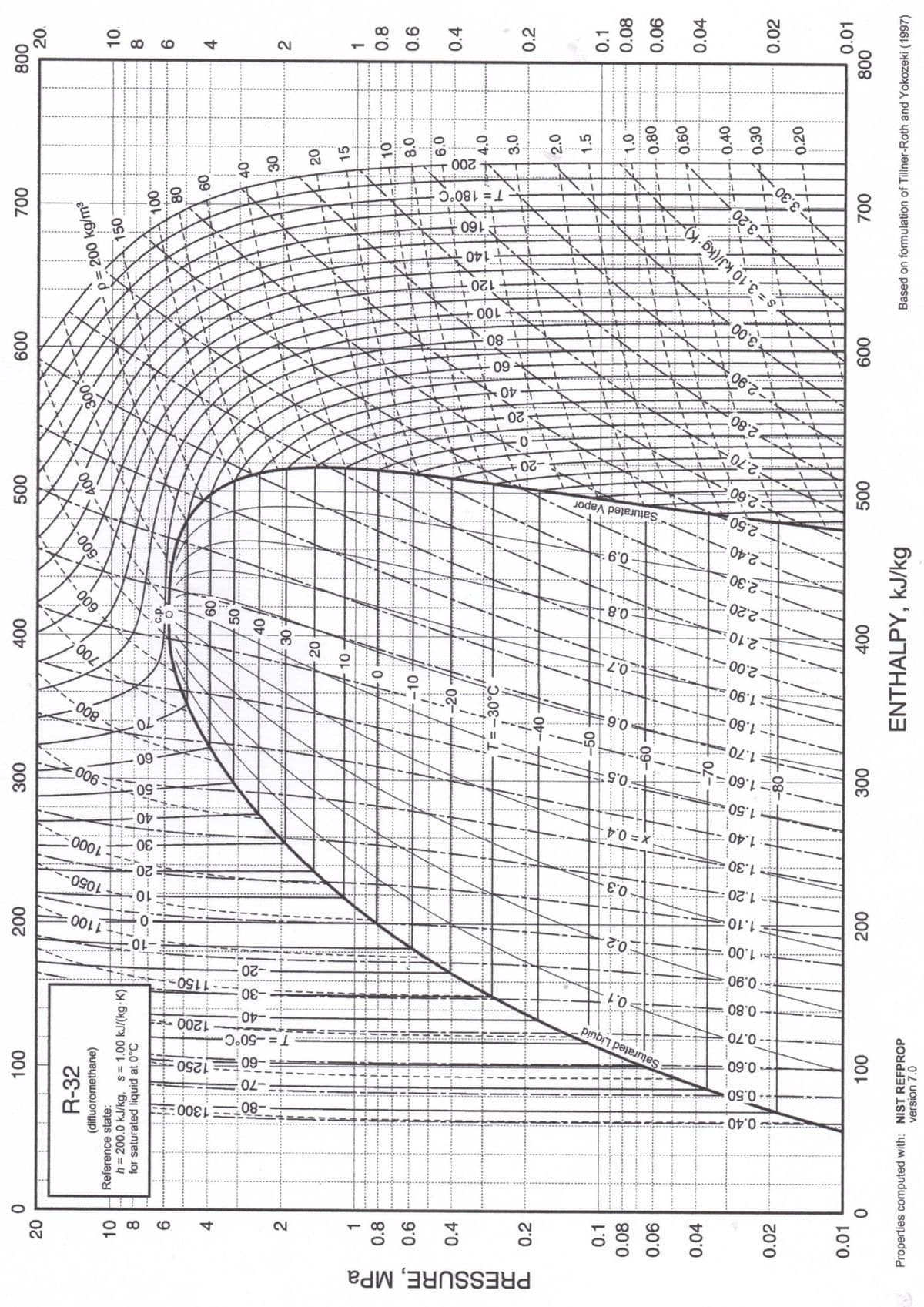
Transcribed Image Text:PRESSURE, MPa
20
10
8
6
4
2
1
0.8
0.6
0.4
0.2
0.1
0.08.
0.06
0.04
0.02
0.01
0
0
R-32
(difluoromethane)
100
Reference state:
h = 200.0 kJ/kg, s= 1.00 kJ/(kg-K)
for saturated liquid at 0°C
1300:
-0.40
+0.50
1250:
-80
-70;
-60
-50°C
-40
-30
-20
I
100
0.60
-0.70
1200
Properties computed with: NIST REFPROP
version 7.0
Saturated Liquid
1150-
+0.1
200
0.2
1100:
-10-
0
10
0.80
-0.90
1.00
1.10
200
1050-
0.3-
1000
1.20
1.30
X = 0.4
88/9 8 849
1.40
300
1.50
006
0.5%
1.60-0
-80
-60
300
SOL
-50
T=-30°C
-40
0.6
-008
-1.80
-10
1.90
0
10
0.7
400
2.00-
700,
20
30
40
-2.10
600
400
20
50
ㅎ 888 엉...
0.8
500-
W
0.9
2.20-1
2.30
-2.40
ENTHALPY, KJ/kg
2.50
500
400
Saturated Vapor
500
-20:
300
20
2.80
+A--
77
X
40
600
60
2.90
A
K
H
The
A
ai
i
TR
600
AN
15
+-+-₂
7.
80
100
4
3.00
S
p = 200 kg/m³
150
V
1
120-
+140
----
A
M
= 3.10 kJ/(kg.
160
·K)₂
S
700
100-
1
T
3.20
08
X
60
T = 180°C
3.30
700
1.
40
30
20
15
002.
10
8.0
6.0
4.0
3.0
2.0
1.5
1.0
0.80
0.60
0.40
0.30
0.20
800
20.
10.
8
6
2
1
0.8
0.6
0.4
0.2
0.1
0.08
0.06
0.04
0.02
0.01
800
Based on formulation of Tillner-Roth and Yokozeki (1997)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning