0.5 kg of steam contained in piston -cylinder device as shown below undergoes expansion from state 1 to state 2. Initial specific internal energy of steam is 2709.9 kJkg The paddle wheel is a device tumed 6270 revolutions with an average of turning moment against the steam is 1955SN mm. A beating device supplies 80 kJ of energy to the steam. Meanwhile, The paddle wheel work and heat source together moves out 0.85 m diameter frictionless piston for a distance of 145 m against a constant pressure of 1.5bar. Determine the following Network of the system and state weather increase or decrease 1) Final internal energy of the steam 1) State and explain, the law of thermodynamics used to find final internal energy of the steam 0.5 kg ar Steam E paddle Piston U=2709.9 kJ/kg Q =80 kJ
0.5 kg of steam contained in piston -cylinder device as shown below undergoes expansion from state 1 to state 2. Initial specific internal energy of steam is 2709.9 kJkg The paddle wheel is a device tumed 6270 revolutions with an average of turning moment against the steam is 1955SN mm. A beating device supplies 80 kJ of energy to the steam. Meanwhile, The paddle wheel work and heat source together moves out 0.85 m diameter frictionless piston for a distance of 145 m against a constant pressure of 1.5bar. Determine the following Network of the system and state weather increase or decrease 1) Final internal energy of the steam 1) State and explain, the law of thermodynamics used to find final internal energy of the steam 0.5 kg ar Steam E paddle Piston U=2709.9 kJ/kg Q =80 kJ
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter2: Steady Heat Conduction
Section: Chapter Questions
Problem 2.39P: The tip of a soldering iron consists of a 0.6-cm- diameter copper rod, 7.6 cm long. If the tip must...
Related questions
Question
need all parts soon to get upvote
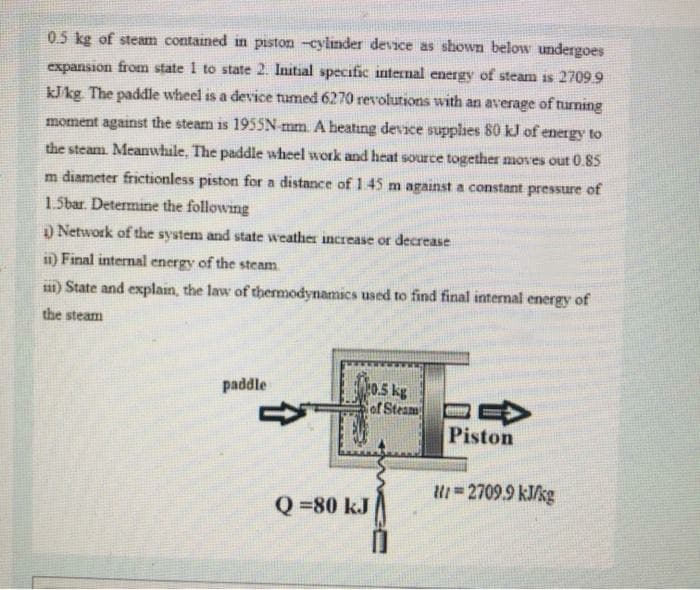
Transcribed Image Text:05 kg of stearm contained in piston -cylinder device as shown below undergoes
expansion from state 1 to state 2. Initial specific internal energy of steam is 2709.9
kJ/kg The paddle wheel is a device tumed 6270 revolutions with an average of turning
moment against the steam is 1955N mm. A heatıng device supplhes 80 kJ of energy to
the steam Meanwhile, The paddle wheel work and heat source together moves out 0.85
m diameter frictionless piston for a distance of145 m against a constant pressure of
1.5bar. Determine the following
) Network of the system and state weather increase or decrease
1) Final internal energy of the steam
a1) State and explain, the law of thermodynamics used to find final internal energy of
the steam
0.5 kg
of Steam
paddle
Piston
U=2709.9 kJ/kg
Q =80 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning