Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and e xplain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Use the following image to determine which statement is true:
-
Calcium is the most reactive because it has the most energy levels (shells.)
-
Carbon is the most reactive because it can take or give 4 electrons.
-
Helium is the most reactive because it is the simplest and can therefor react easier.
-
Lithium is the most reactive because it has a valence of 1.
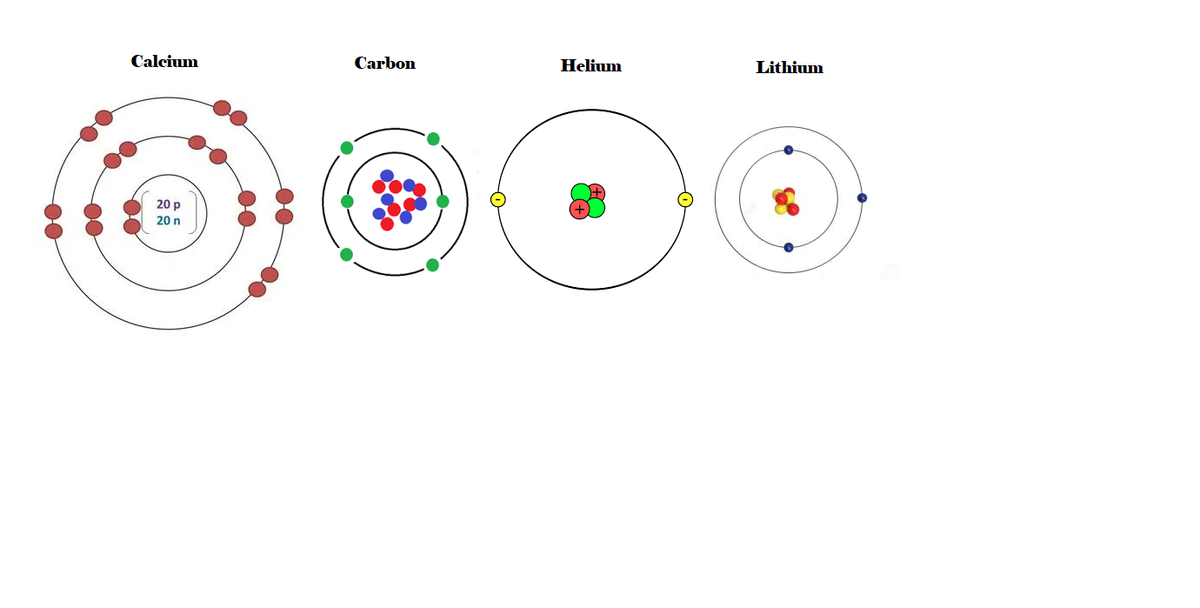
Transcribed Image Text:00
Calcium
Carbon
Helium
Lithium
20 p
20 n
-O-
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
