Chapter5: Stereochemistry At Tetrahedral Centers
Section5.SE: Something Extra
Problem 58AP: One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This...
Related questions
Question
Draw the most important resonance structure of the cationic intermediate leading to the major product of electrophilic bromination of the compound below
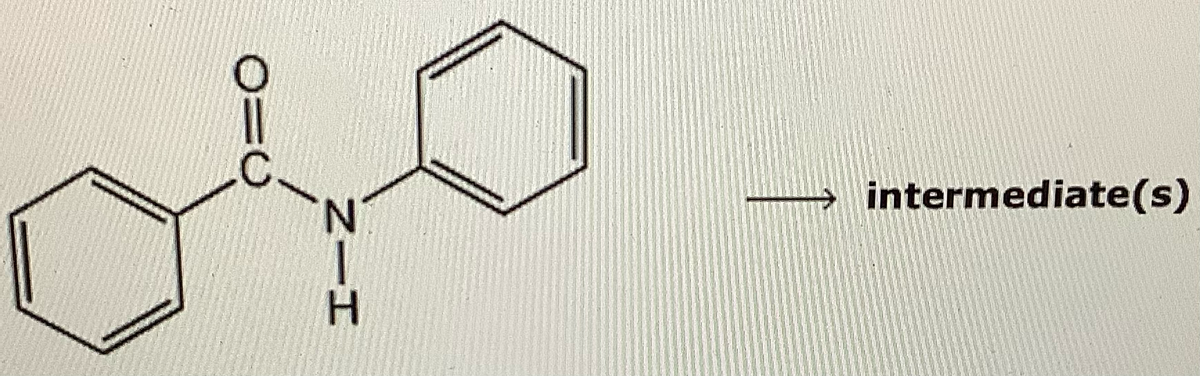
Transcribed Image Text:O=C
N.
intermediate(s)
Expert Solution
Step 1
Solution - Bromination of benzanilide is electrophilic addition of Br+ to aromatic ring . Ortho and para products are formed and ortho product is steric ally hindered as compared to para product so para product is electron rich as compared to ortho product . Hence major product is para product.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning