02% hydrogen. What is the empirical formula of this co mical notation is case sensitive ur chemical notation here nass of this compound is 96.94 g/mol, what is its mole
02% hydrogen. What is the empirical formula of this co mical notation is case sensitive ur chemical notation here nass of this compound is 96.94 g/mol, what is its mole
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 3ALQ: True or false? The atom with the largest subscript in a formula is die atom with the largest percent...
Related questions
Question
Need help solving this
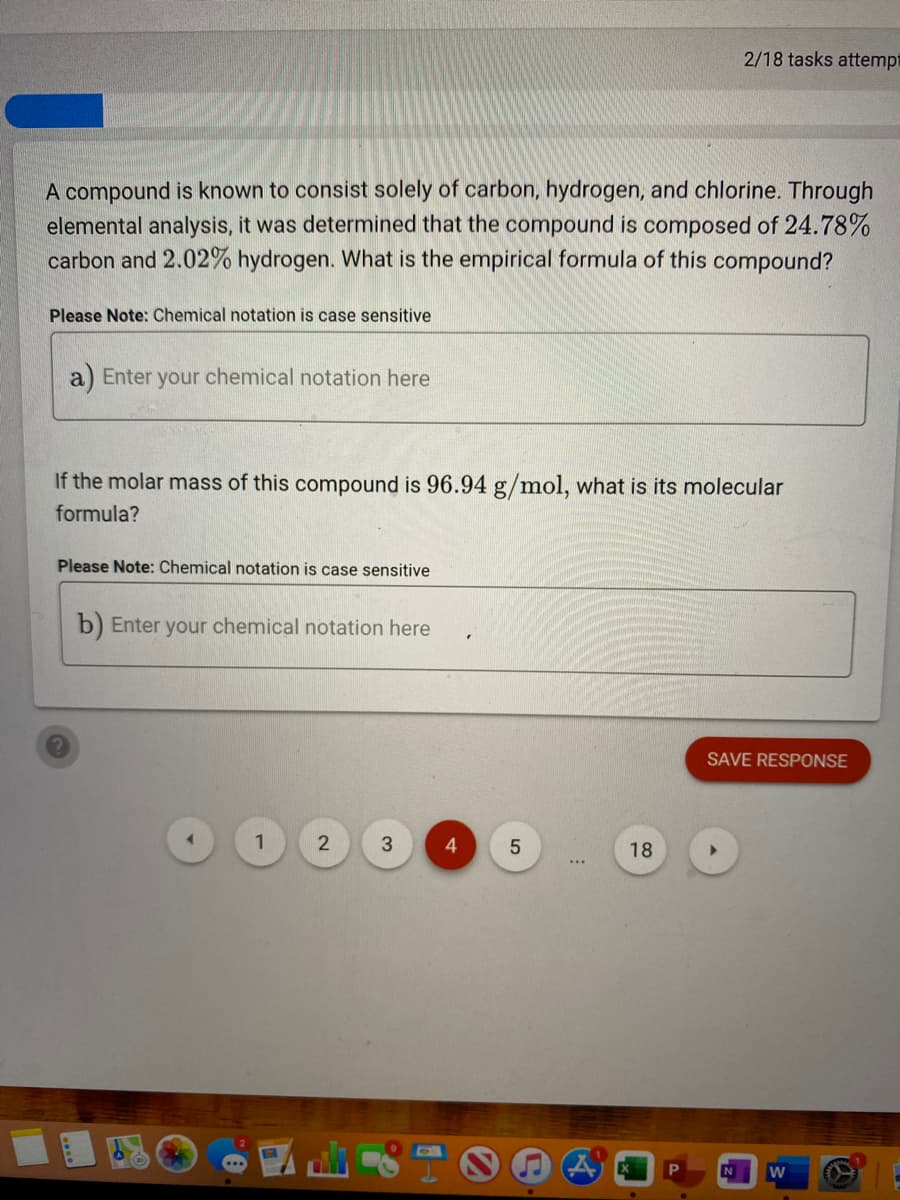
Transcribed Image Text:2/18 tasks attempt
A compound is known to consist solely of carbon, hydrogen, and chlorine. Through
elemental analysis, it was determined that the compound is composed of 24.78%
carbon and 2.02% hydrogen. What is the empirical formula of this compound?
Please Note: Chemical notation is case sensitive
a) Enter your chemical notation here
If the molar mass of this compound is 96.94 g/mol, what is its molecular
formula?
Please Note: Chemical notation is case sensitive
b) Enter your chemical notation here
SAVE RESPONSE
4.
18
...
N
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.