1 2 3 The enthalpy of combustion for 3 moles of ethane at SATP conditions is negative Type your answer... kJ. Which one of the following would cause 100mL of water to cool by 5.0°C in an isolated system? The decomposition of 0.19mol of N₂O4(g) The formation of 0.19mol of N₂O4(g) The decomposition of 9.2mol of ethyne gas The formation of 9.2mol of ethyne gas Please use the following thermochemical equation for the next question. 2 A+ B₂ 3C + 150 kJ The AH for the reaction 6C4A + 2 B2 would be: +150 kJ - 150 kJ +300 kJ - 300 kJ
1 2 3 The enthalpy of combustion for 3 moles of ethane at SATP conditions is negative Type your answer... kJ. Which one of the following would cause 100mL of water to cool by 5.0°C in an isolated system? The decomposition of 0.19mol of N₂O4(g) The formation of 0.19mol of N₂O4(g) The decomposition of 9.2mol of ethyne gas The formation of 9.2mol of ethyne gas Please use the following thermochemical equation for the next question. 2 A+ B₂ 3C + 150 kJ The AH for the reaction 6C4A + 2 B2 would be: +150 kJ - 150 kJ +300 kJ - 300 kJ
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.85QE: The octane number of gasoline is based on a comparison of the gasolines behavior with that of...
Related questions
Question
Chemistry
Hello,
Please ONLY attempt if you intend to try all of the following questions.
Thank you.
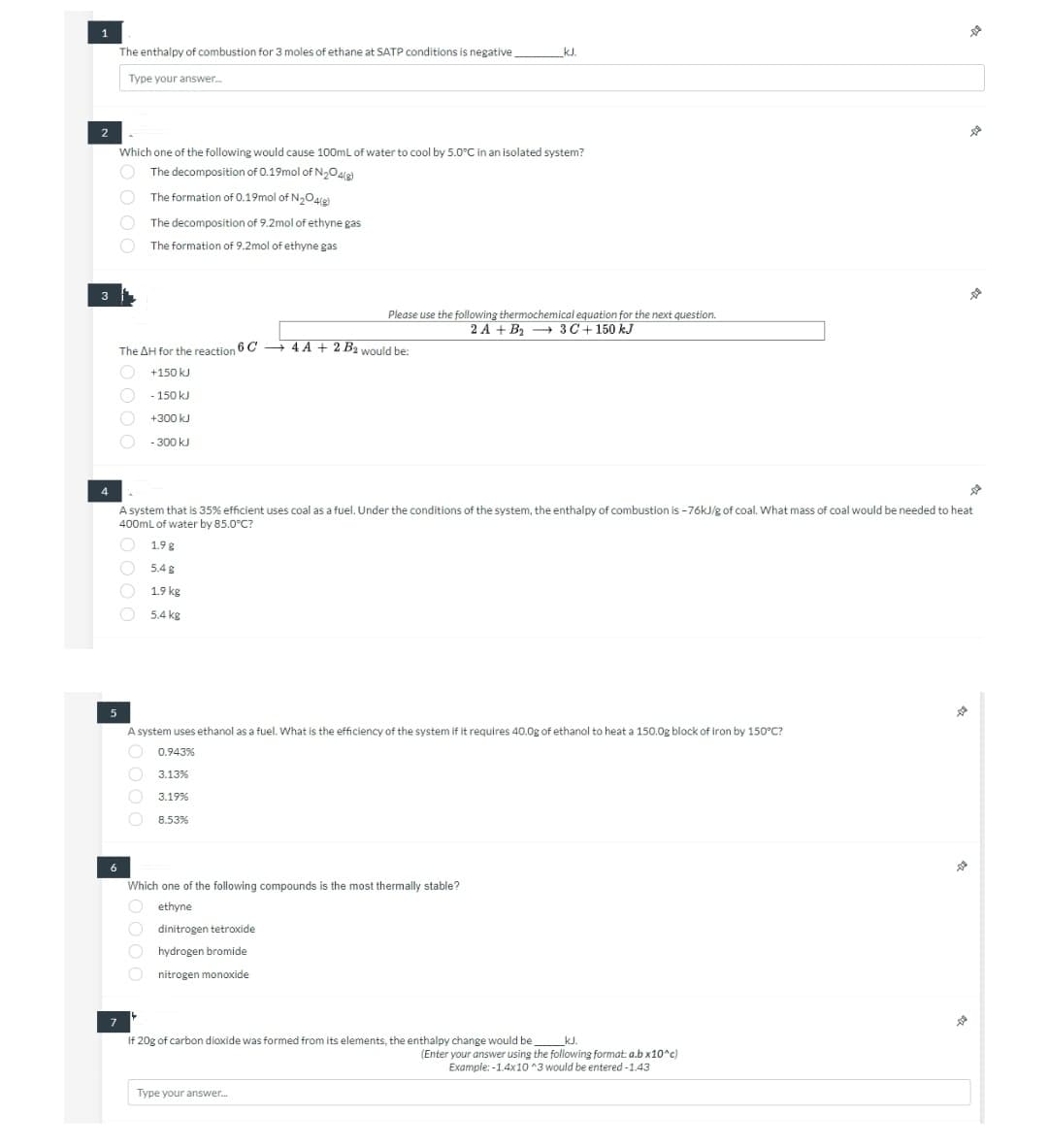
Transcribed Image Text:1
2
3
4
5
The enthalpy of combustion for 3 moles of ethane at SATP conditions is negative.
Type your answer.....
6
Which one of the following would cause 100mL of water to cool by 5.0°C in an isolated system?
The decomposition of 0.19mol of N₂O4(8)
The formation of 0.19mol of N₂O4(g)
C
The AH for the reaction 6C4A + 2 B2 would be:
+150 kJ
- 150 kJ
+300 kJ
- 300 kJ
C
10000
The decomposition of 9.2mol of ethyne gas
The formation of 9.2mol of ethyne gas
A system that is 35% efficient uses coal as a fuel. Under the conditions of the system, the enthalpy of combustion is -76kJ/g of coal. What mass of coal would be needed to heat
400mL of water by 85.0°C?
1.9 g
5.4 g
1.9 kg
5.4 kg
C
Please use the following thermochemical equation for the next question.
2 A+ B₂ 3 C + 150 kJ
A system uses ethanol as a fuel. What is the efficiency of the system if it requires 40.0g of ethanol to heat a 150.0g block of iron by 150°C?
0.943%
3.13%
O
3.19%
O 8.53%
Which one of the following compounds is the most thermally stable?
ethyne
_kJ.
dinitrogen tetroxide
hydrogen bromide
nitrogen monoxide
7
If 20g of carbon dioxide was formed from its elements, the enthalpy change would be
Type your answer...
D
_kJ.
(Enter your answer using the following format: a.b x10^c)
Example: -1.4x10^3 would be entered -1.43
De
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
