Q: select the labels that apply to each of the following pairs of coordination complexes.
A: Stereoisomers : isomers those have different arrengement in 3D space but have same bonding…
Q: The total ionic equation for the reaction observed in this week's lab is: H*(aq) + Cl(aq) + Na*(aq)…
A:
Q: match the following statements with the correct term (descriptor): Mixing of the 2s atomic orbital…
A: Molecular orbital is formed by the combination of atomic orbitals. In the given question, it has…
Q: Please help me with the mechanism for what is described below: 1. Draw a mechanism for the…
A: The above question we have to draw the mechanism for Wittig reaction and discuss the product…
Q: What is the smallest possible value of the principal quantum number n for a p electron? 17=
A: According to quantum mechanics, there are four types of quantum numbers. The electrons are filled in…
Q: An atomic cation with a charge of +1 has the following electron configuration: [Ne] 3s²3 p¹ What is…
A: Here the electronic configuration of the cation is : [Ne] 3S²3P¹ Also the element is in +1 oxidation…
Q: A VHF television station assigned to channel 12 transmits its signal using radio waves with a…
A: Given, Frequency of the radio wave is, ν=204 MHz = 204×106 Hz ν=204×106 Hz = 204×106 s-1 {…
Q: Identify which of the following reactions are redox reactions. Choose one or more: NaHCO3(aq) +HI…
A: Given,The reactions:NaHCO3(aq) + HI(aq)→ NaI(aq) + H2O(l) + CO2(g)NaCl(aq) + AgNO3(aq)→ NaNO3(aq) +…
Q: HN N=
A: The oxidation number represents the loss or gain of electrons to the respective elements of the…
Q: 1.) The molar heat of combustion of octane to form carbon dioxide and water vapour is ─5470.1kJ/mol.…
A: “Since you have posted multiple questions, we will provide the solution only to the first question…
Q: Thought questions to help you with the selective precipitation related questions (don't spend too…
A: Note: Since you have posted a question with multiple sub-parts, we will provide the solution only to…
Q: Step 1 Draw the missing curved arrow(s) for step one of the mechanism (first elimination). Add any…
A: Amide is a base it abstracts proton from the vicinal dihalides followed by elimination of Halide ion…
Q: 5. How can chemistry help in nanotechnology?
A: Chemistry plays a crucial role in nanotechnology, providing the necessary principles and tools for…
Q: Write the resulting linear regression equation and R² of the plotted standard calibration curve.
A: To provide the regression equation and the R2 value of the plotted standard calibration curve.
Q: What is the IUPAC name of the following compound?
A: A question based on atomic models. A ball and stick model representation of a molecule is given…
Q: Select the major product of the following reaction. O₂N 1. Cly/FeCly 2. HNO₂/ H₂SO, 3. Cly/FeCly 4.…
A: Unlike alkenes, benzene and other Aromatic compounds do not undergo addition reactions . Addition of…
Q: If a solution of sucrose (C12H22O11) has a molarity of 0.245 how many milliliters of solution are…
A: Given,Molarity of sucrose ( C12H22O11 ) =0.245 Mmass of sucrose = 50.0 g.
Q: Determine the number of electrons contained in the rings of each structure. Drag the appropriate…
A: A question based on introduction to organic chemistry. 5 different organic compounds are given that…
Q: 17. Devise a short synthesis of compound A from acetoacetic ester. Consider using Wittig olefination…
A: an organic chemical reaction wherein an aldehyde or a ketone is reacted with a Wittig Reagent (a…
Q: Based only on their intermolecular forces, which of the following solutions would be immiscible in…
A: Substances with similar intermolecular attractive forces tend to be soluble in one another. i.e.…
Q: This question will walk you through the process of calculating the number of atoms present in a…
A:
Q: Q18: The pH scale is a range from: A) 1-7 B) 0-14 C) 1-14 D) 1-20 E) 0-7
A: Detail The pH scale range is given below.
Q: esc A few different types of electromagnetic radiation are listed in the table below. Complete the…
A: We have been given four types of radiations: Green light, X-rays,ultraviolet radiation and…
Q: Combustion of glucose (C6H₁2O6) is the main source of energy for animal cells: C6H₁2O6(s) + 60₂(g) →…
A:
Q: What is the major product after addition of two HF to propyne, CH3C=CH? a) CH3CHFCH₂F b) CH₂FCH₂CH₂F…
A: Detail
Q: Cu(NH3)4]2+ forms a blue solution. When concentrated HCl is added to this solution, what color will…
A: Hydrochloric acid (HCl) is a strong corrosive acid which reacts with the compounds easily. The…
Q: 25 mL of hydroiodic acid generates 1.20 L of H2 gas at STP conditions when combined with excess…
A: Data
Q: 1. Describe what happens in a substitution reaction. Identify a step in the processes shown in which…
A: We are given synthesis of aspirin from the oil of wintergreen and we have to identify the…
Q: Please help me with the mechanism below: 1. Draw the mechanism for the reaction described here -…
A: The above question is based on organic reaction mechanism . We have to discuss about the mechanism…
Q: An electron in an atom is known to be in a state with magnetic quantum number m₁ =2. What is the…
A: • Principal quantum number (n): Determine the orbital size and an energy level an electron is…
Q: The SUM of the formal charges for the following (nonexistent) compound is [answer1]. Use the…
A: 1. In a compound exist more than one Lewis structure is possible, the concept of formal charge…
Q: Consider the following data on some weak acids and weak bases: hydrofluoric acid name nitrous acid…
A: We need to arrange the given solutions in order of increasing pH based on the given Ka and Kb…
Q: Write the empirical formula of at least four binary ionic compounds that could be formed from the…
A:
Q: For the radical bromination of cyclohexane Initiation : B12 hv Give the following mechanism using…
A: Detail
Q: An atom of helium has a radius He = 31. pm and an average speed in the gas phase at 25°C of 787.m/s.…
A: According to Heisenberg uncertainty principle, position (x) and momentum of any microscopic particle…
Q: Is the indicated chiral center R or S? HO, R 20 OS OH OH OH CH₂OH
A: Stereochemistry is branch of chemistry in which we deal with three dimensional arrangement of atoms…
Q: Calculate (in MeV) the nuclear binding energy for a vanadium-52 nuclide. The atomic mass of this…
A: To calculate the nuclear binding energy for a vanadium-52 nuclide , we need to know the mass defect…
Q: 3. Based on their molecular formulas, which of the following are NOT carbohydrates? Saccharides have…
A: Saccharides have a general formula of (CH2O)n n=3-9, the most common being 5,6 glucose and fructose…
Q: The preparations of two aqueous solutions are described in the table below. For each solution, write…
A: In an aqueous solution, an acid is described as a substance that produces [H+] hydronium ions while…
Q: Draw the coupling product of the Pd(0)-catalyzed Suzuki reaction between the following compounds.…
A: We are given reactants for the Pd(0)-catalyzed Suzuki reaction. This is the cross coupling between…
Q: Suppose you start with a solution of red dye #40 that is 2.5 ✕ 10−5 M. If you do four successive…
A: Molarity => The molarity is measure of the Concentration of any solute in per unit Volume of…
Q: 45. Write the half-reactions and cell reaction occurring during electrolysis of each molten salt…
A: Now from the Farady's law of electrolysis, M = F×m×nQ [ where m = molar mass of the substance, F =…
Q: GenAlex Medical, a leading manufacturer of medical laboratory equipment, is designing a new…
A: It is based on the concept of concentration. Here we are required find the mass of digoxin that can…
Q: CHS - 1 CH3 H₂C OOK 11 CH₂ IV -CH3 4 O₂N Your Aktiv Learning trial expires on 05/23/23 at 12:59 AM…
A:
Q: Specify the hybridization at the atoms labelled a-d. For each atom enter one of the following: sp3,…
A:
Q: 2 H₂O Bond Bond total bond energy for the reactants in the energy (kJ/mol) Average Bond Energies…
A: The reaction : C2H4 + 3O2 →2CO2 +2H2O H2-C=C-H2 +3O=O →2O=C=O +2 H-O-H
Q: H3C CH3
A: Using butanol i.e alcohol contains 4 carbon atom treated with kmno4 or cro3 this are oxidizing…
Q: Select the major product of the following reaction. OCH₂ & OCH3 OCH3 & S Br Br OCH3 E Br₂/ FeBr3 Br…
A: Unlike alkenes, benzene and other aromatic compounds do not give addition reaction . Addition of…
Q: 6. (a) Draw Lewis structures and MO diagrams for CN*, CN, and CN™. Compound Lewis Diagram CN* CN CN…
A: Lewis model is used to represent the valence shell electron's arrangement around an individual atom…
Q: H₂ -N H OH OH DMSO (1 equiv.) Oxalyl chloride (1 equiv.) CH₂Cl₂, -78 °C OsO4 (cat.) NMO (1 equiv.)…
A:
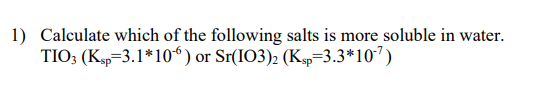
Step by step
Solved in 3 steps with 3 images

- What is the concentration of Cd2+ in a 0.016 M Cd(NO3)2 solution that is also 1.0 M NH3? For Cd(NH3)42+, Kf = 1.0 x 107.gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.(a) BaSiF6, 0.026 g/100 mL (contains SiF6 2− ions)(b) Ce(IO3)4, 1.5 × 10–2 g/100 mL(c) Gd2(SO4)3, 3.98 g/100 mL(d) (NH4)2PtBr6, 0.59 g/100 mL (contains PtBr6 2− ions)(a) Explain why Mg(OH)2 precipitates when CO32- ion isadded to a solution containing Mg2+. (b) Will Mg(OH)2precipitate when 4.0 g of Na2CO3 is added to 1.00 L of asolution containing 125 ppm of Mg2+?
- For the aqueOuS [Ni(NH3)6] 2+ complex Kf= 5.50 x 10^8 at 25 °C. Suppose equal volumes of 0.0016 M Ni(NO3)2 solution and 0.28 M NH3 solution are mixed. Calculate the equilibrium molarity of aqueous Ni2+ ion. Round your answer to 2 significant digits.7.00 grams of Cobalt (II) nitrate are dissolved in 1.00 liter of water. What is the initial concentration of CO (2+) in moles per liter? Then, 0.22 moles of solid KOH are added to this solution. The solution volume remains 1.00 liter. Given that Kf = 5.0 x 10^9 for the complex ion, Co(OH)4 (2-) find the final concentrations of Co (2+), Co(OH)4 (2-), and OH-. Also find the pH of this final solution.For the aqueous [Cu(NH3)4]^2+ complex =Kf=5.6x10^11 at 25°C.Suppose equal volumes of 0.0058M Cu(NO3)2 solution and 0.66M NH3 solution are mixed. Calculate the equilibrium molarity of aqueous Cu^2+ ion.Round your answer to 2 significant digits.
- Use crystal field theory to explain why ionic radii of Fe2+ and Co2+ are smaller than expected.For the aqueous [Cu(NH3)4 ] 2+ complex kf =5.6 x1011 at25°C .Suppose equal volumes of 0.0062M Cu(NO3)2 solution and 0.88M NH3 solution are mixed. Calculate the equilibrium molarity of aqueous Cu2+ ion.Round your answer to 2 significant digits.Which member of each pair produces the more acidic aqueous solution: (a) ZnBr2 or CdCl2, (b) CuCl or Cu(NO3)2, (c)Ca(NO3)2 or NiBr2? Explain.
- (a) In early studies it was observed that when the complex [Co(NH3)4Br2]Br was placed in water, the electrical conductivityof a 0.05 M solution changed from an initial value of191 ohm-1 to a final value of 374 ohm-1 over a period of anhour or so. Suggest an explanation for the observed results.(b) Write a balanced chemical equation to describe the reaction.(c) A 500-mL solution is made up by dissolving 3.87 g ofthe complex. As soon as the solution is formed, and beforeany change in conductivity has occurred, a 25.00-mLportion of the solution is titrated with 0.0100 M AgNO3solution. What volume of AgNO3 solution do you expectto be required to precipitate the free Br-(aq)? (d) Based onthe response you gave to part (b), what volume of AgNO3 solution would be required to titrate a fresh 25.00-mL sampleof [Co(NH3)4Br2]Br after all conductivity changes haveoccurred?For the aqueous [Ni(NH3 )6]2+ complex Kf=5.50 x 108 at 25C . Suppose equal volumes of 0.0024 M Ni(NO3 )2solution 0.94M NH3solution are mixed. Calculate the equilibrium molarity of aqueous Ni2+ ion. Round your answer to 2 significant digits.The hardness of water is usually expressed in parts per million (ppm) by mass of CaCO3. What is the hardness of a water sample with a Ca2+ ion concentration of 1.1×10-3 M?
