Chapter2: Polar Covalent Bonds; Acids And Bases
Section2.SE: Something Extra
Problem 25MP: Use curved arrows to draw the protonated form of each Lewis base below.
Related questions
Question
100%
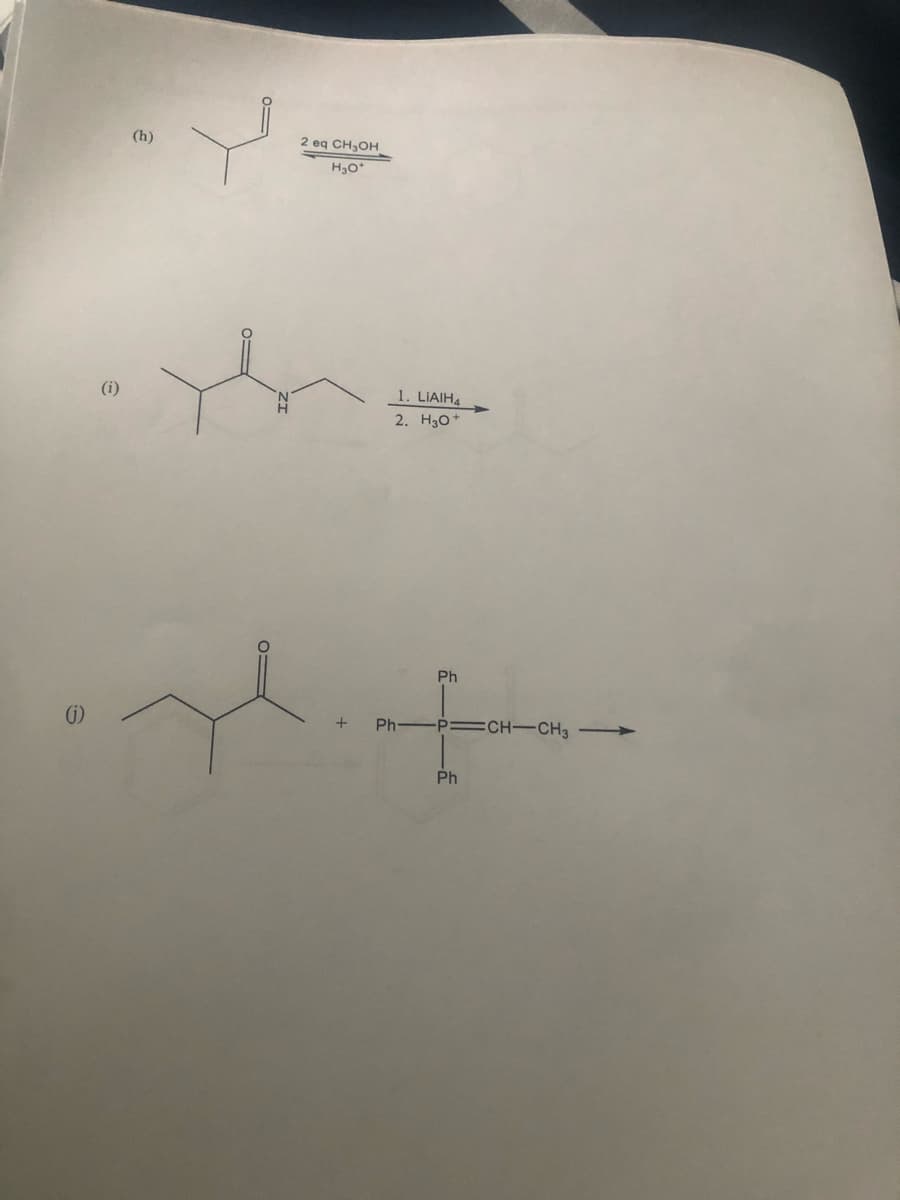
Transcribed Image Text:(h)
2 eq CH₂OH
H₂O*
+
Ph
1. LIAIH4
2. H3O+
Ph
-P=CH-CH3
Ph
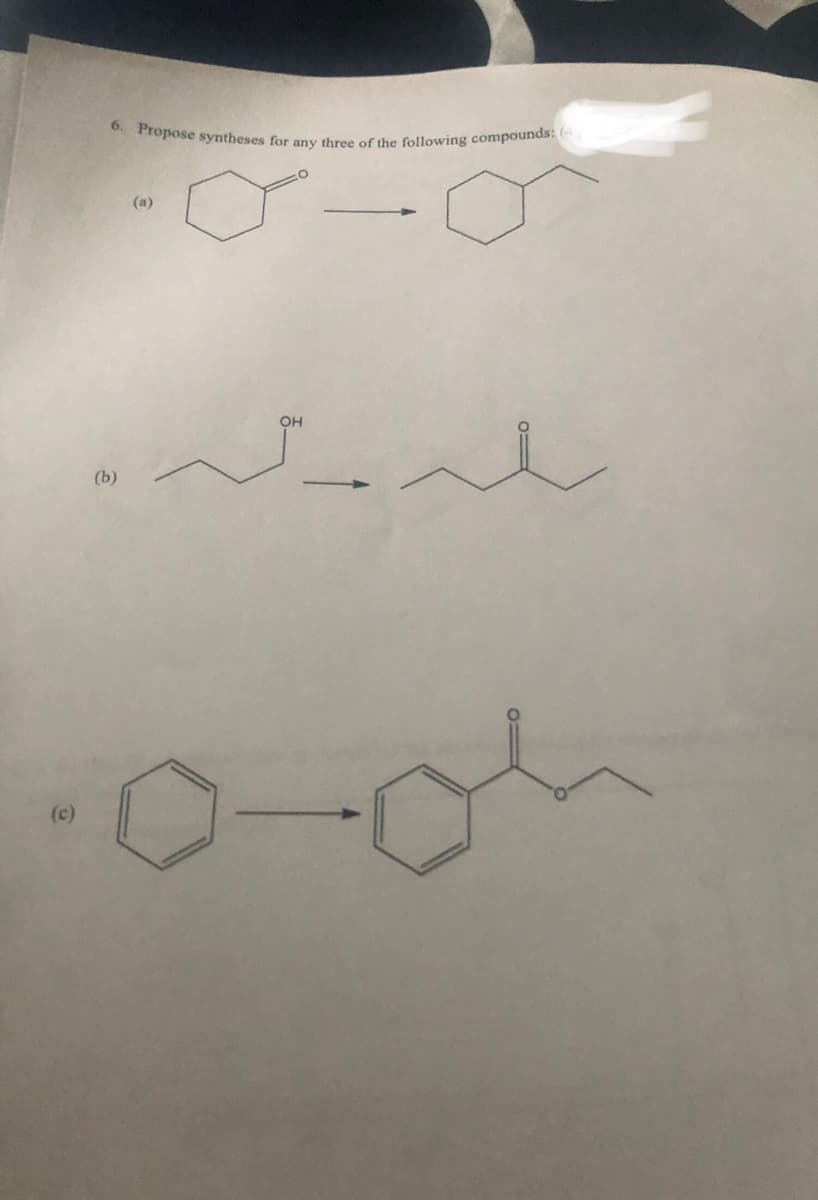
Transcribed Image Text:(c)
6. Propose syntheses for any three of the following compounds: (4
(a)
بلدهید
(b)
OH
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



