* 1 point 22. When an inflated balloon experiences a decrease in size, the air pressure inside the balloon remains nearly constant. If there is no transfer of energy by heat to or from the balloon, what physical change takes place in the balloon? The average potential energy of the gas particles increases, so the balloon becomes hotter. The average potential energy of the gas particles decreases, so the balloon becomes colder. The average kinetic energy of the gas particles increases, so the balloon becomes hotter. The average kinetic energy of the gas particles decreases, so the balloon becomes colder. 1 point 23. Some amount of energy is transferred by heat into a system. The net work done by the system is 50J, while the increase in its internal energy is 30J. What is the amount of net heat? -80J -20J 80J 20J
* 1 point 22. When an inflated balloon experiences a decrease in size, the air pressure inside the balloon remains nearly constant. If there is no transfer of energy by heat to or from the balloon, what physical change takes place in the balloon? The average potential energy of the gas particles increases, so the balloon becomes hotter. The average potential energy of the gas particles decreases, so the balloon becomes colder. The average kinetic energy of the gas particles increases, so the balloon becomes hotter. The average kinetic energy of the gas particles decreases, so the balloon becomes colder. 1 point 23. Some amount of energy is transferred by heat into a system. The net work done by the system is 50J, while the increase in its internal energy is 30J. What is the amount of net heat? -80J -20J 80J 20J
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter21: Heat Engines, Entropy, And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 45AP
Related questions
Question
22-23.Answer
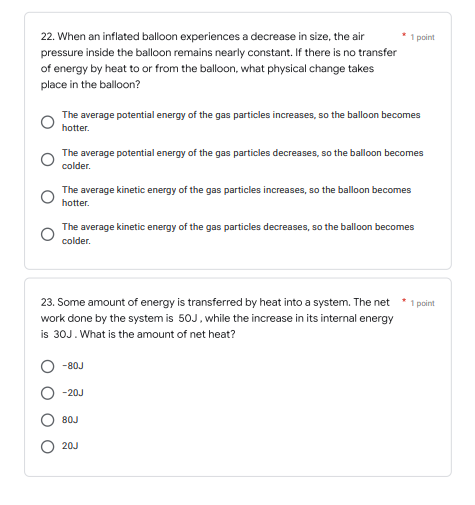
Transcribed Image Text:22. When an inflated balloon experiences a decrease in size, the air
pressure inside the balloon remains nearly constant. If there is no transfer
of energy by heat to or from the balloon, what physical change takes
place in the balloon?
The average potential energy of the gas particles increases, so the balloon becomes
hotter.
The average potential energy of the gas particles decreases, so the balloon becomes
colder.
The average kinetic energy of the gas particles increases, so the balloon becomes
hotter.
The average kinetic energy of the gas particles decreases, so the balloon becomes
colder.
*
1 point
23. Some amount of energy is transferred by heat into a system. The net
work done by the system is 50J, while the increase in its internal energy
is 30J. What is the amount of net heat?
-80J
-20J
80J
20J
1 point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning