1 What is the symbol for the enthalpy change that occurs during the phase change of a substance from solid to liquid? A. ΔΗ'melt B. ΔΗre C. ΔΗ50il D. ΔΗ"νap E. AH°cond You are building an ice skating rink and want to select flooring that experiences the least amount of temperature shift under changing conditions. Which substance would be least suitable, based on specific heat cap.? A. aluminum B. concrete C. granite D. wood
1 What is the symbol for the enthalpy change that occurs during the phase change of a substance from solid to liquid? A. ΔΗ'melt B. ΔΗre C. ΔΗ50il D. ΔΗ"νap E. AH°cond You are building an ice skating rink and want to select flooring that experiences the least amount of temperature shift under changing conditions. Which substance would be least suitable, based on specific heat cap.? A. aluminum B. concrete C. granite D. wood
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 109SCQ
Related questions
Question
Multiple Choice
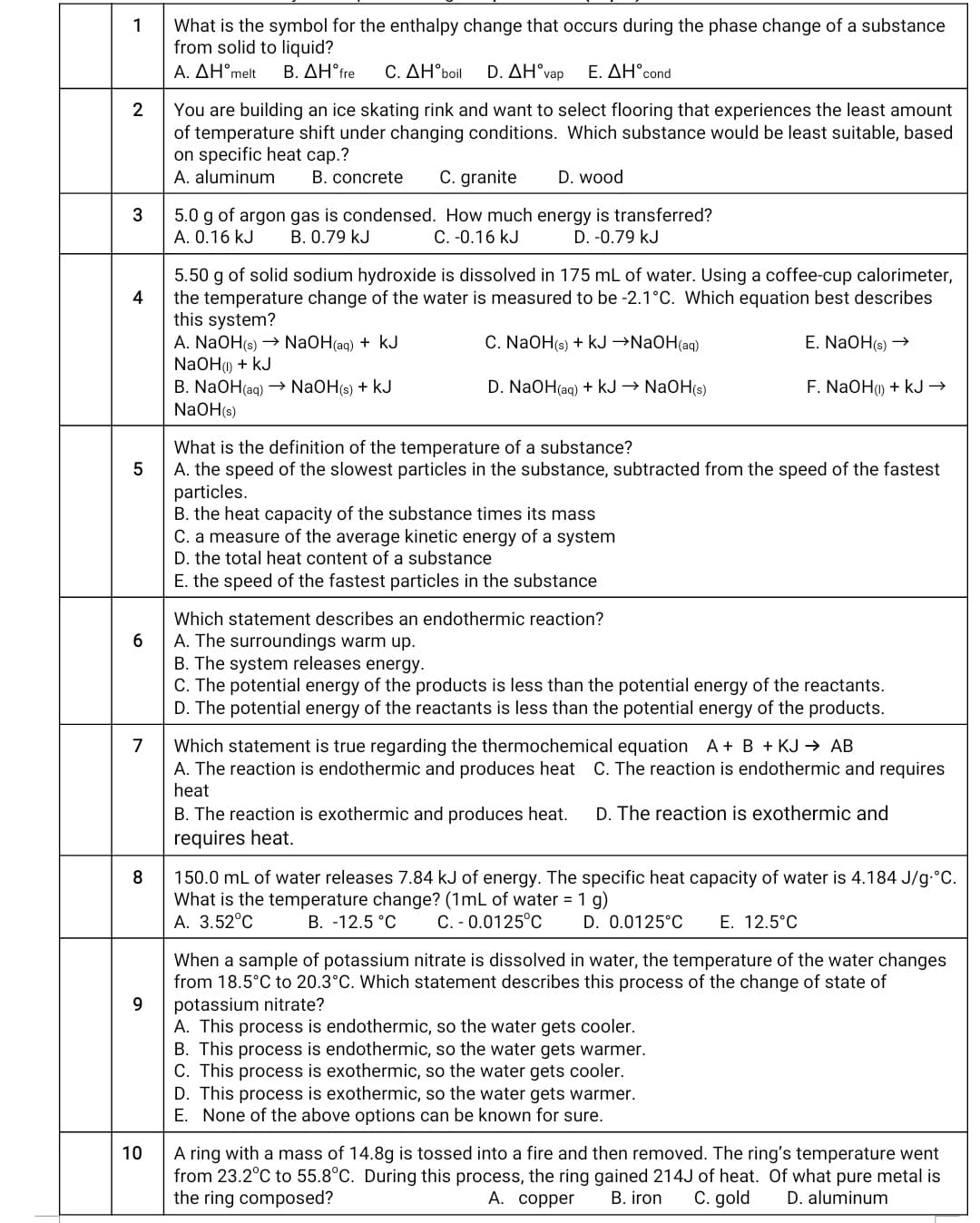
Transcribed Image Text:1
What is the symbol for the enthalpy change that occurs during the phase change of a substance
from solid to liquid?
A. ΔΗ'Μelt
B. ΔΗfre
C. ΔΗ"boil
D. AH°vap
E. ΔΗ'cond
You are building an ice skating rink and want to select flooring that experiences the least amount
of temperature shift under changing conditions. Which substance would be least suitable, based
on specific heat cap.?
A. aluminum
B. concrete
C. granite
D. wood
3
5.0 g of argon gas is condensed. How much energy is transferred?
A. 0.16 kJ
B. 0.79 kJ
C. -0.16 kJ
D. -0.79 kJ
5.50 g of solid sodium hydroxide is dissolved in 175 mL of water. Using a coffee-cup calorimeter,
the temperature change of the water is measured to be -2.1°C. Which equation best describes
this system?
A. NaOH(s) → NaOH(aq) + kJ
NaOH) + kJ
B. NaOH(aq) → NaOH(s) + kJ
NaOH(s)
4
C. NaOH(s) + kJ →NAOH(ag)
E. NaOH(s) →
D. NaOH(ag) + kJ → NaOH(s)
F. NaOH) + kJ →
What is the definition of the temperature of a substance?
5
A. the speed of the slowest particles in the substance, subtracted from the speed of the fastest
particles.
B. the heat capacity of the substance times its mass
C. a measure of the average kinetic energy of a system
D. the total heat content of a substance
E. the speed of the fastest particles in the substance
Which statement describes an endothermic reaction?
A. The surroundings warm up.
B. The system releases energy.
C. The potential energy of the products is less than the potential energy of the reactants.
D. The potential energy of the reactants is less than the potential energy of the products.
6
Which statement is true regarding the thermochemical equation A + B + KJ → AB
A. The reaction is endothermic and produces heat C. The reaction is endothermic and requires
heat
7
B. The reaction is exothermic and produces heat.
D. The reaction is exothermic and
requires heat.
8.
150.0 mL of water releases 7.84 kJ of energy. The specific heat capacity of water is 4.184 J/g-°C.
What is the temperature change? (1mL of water = 1 g)
A. 3.52°C
B. -12.5 °C
C. - 0.0125°C
D. 0.0125°C
E. 12.5°C
When a sample of potassium nitrate is dissolved in water, the temperature of the water changes
from 18.5°C to 20.3°C. Which statement describes this process of the change of state of
potassium nitrate?
A. This process is endothermic, so the water gets cooler.
B. This process is endothermic, so the water gets warmer.
C. This process is exothermic, so the water gets cooler.
D. This process is exothermic, so the water gets warmer.
E. None of the above options can be known for sure.
9.
A ring with a mass of 14.8g is tossed into a fire and then removed. The ring's temperature went
from 23.2°C to 55.8°C. During this process, the ring gained 214J of heat. Of what pure metal is
the ring composed?
10
А. соррer
B. iron
C. gold
D. aluminum
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning