1 When epoxides react, they undergo ring-opening by a cleavage of the C-O bond. Why is it easier to break a C-O bond of an epoxide compared to breaking a C-O bond of other ethers? A. A more stable carbocation is formed when an epoxide ring-opens. B. Breaking the epoxide C-O bond relieves the strain of a three-member ring. C. Epoxide ring opening conforms to a faster E2 mechanism. D. Mechanistically, the epoxide carbon always becomes protonated, enhancing it's ring opening reactivity.
1 When epoxides react, they undergo ring-opening by a cleavage of the C-O bond. Why is it easier to break a C-O bond of an epoxide compared to breaking a C-O bond of other ethers? A. A more stable carbocation is formed when an epoxide ring-opens. B. Breaking the epoxide C-O bond relieves the strain of a three-member ring. C. Epoxide ring opening conforms to a faster E2 mechanism. D. Mechanistically, the epoxide carbon always becomes protonated, enhancing it's ring opening reactivity.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter23: Addition To A Carbonyl
Section: Chapter Questions
Problem 42CTQ
Related questions
Question

Transcribed Image Text:1 When epoxides react, they undergo ring-opening by a
cleavage of the C-O bond. Why is it
easier to break a C-O bond of an epoxide compared to
breaking a C-O bond of other ethers?
A. A more stable carbocation is formed when an epoxide
ring-opens.
B. Breaking the epoxide C-O bond relieves the strain of a
three-member ring.
C. Epoxide ring opening conforms to a faster E2
mechanism.
D. Mechanistically, the epoxide carbon always becomes
protonated, enhancing it's ring
opening reactivity.
Expert Solution
Step 1
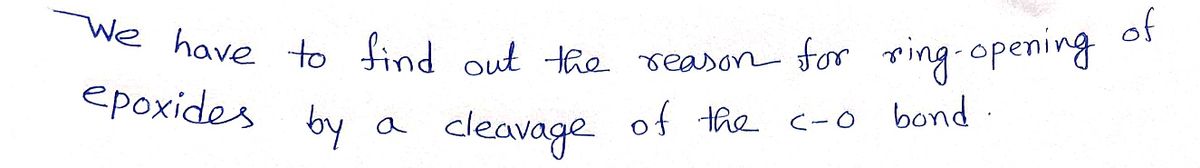
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning