Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 4QAP: Follow the directions of Question 1 for (a) CIF4- (b) PF6- (c) CNS- (d) SnCl5-
Related questions
Question
Show all working explaining detailly each step.
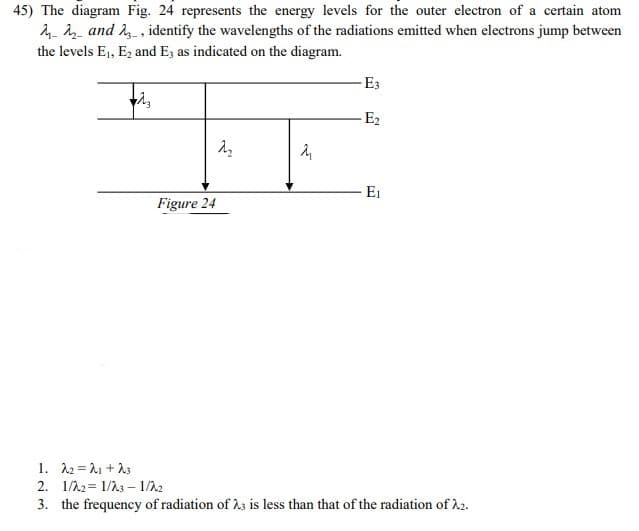
Transcribed Image Text:45) The diagram Fig. 24 represents the energy levels for the outer electron of a certain atom
4- and 2 , identify the wavelengths of the radiations emitted when electrons jump between
the levels E1, E2 and E3 as indicated on the diagram.
E3
-E2
E1
Figure 24
1. 12 = 21 +13
2. 1/22= 1/23 – 1/22
3. the frequency of radiation of h3 is less than that of the radiation of h2.
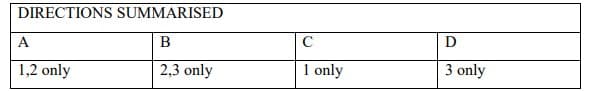
Transcribed Image Text:DIRECTIONS SUMMARISED
A
B
C
D
1,2 only
2,3 only
1 only
3 only
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning