44. Which of the following elements would be expected to show the similar chemical properties based on their electronic configurations? (1) 1s²2s²2p¹ 1s²2s²2p 3s²3p63d¹04s¹ (III) 1s²2s²2p 3s²3p¹ Is²2s²2p 3s²3p³ a. I & II b. I, II & III 45. Which of the following comparisons of atomic radii (size) is incorrect? a) Na < Al b) Cs > Ba 46. Which of the following comparisons of ionization energy is incorrect? a) Na> K b) Ca> K c) Cs > K d) Mg < Al (II) (IV) c. I & III d. III & IV e. none of a to d c) N Ca
44. Which of the following elements would be expected to show the similar chemical properties based on their electronic configurations? (1) 1s²2s²2p¹ 1s²2s²2p 3s²3p63d¹04s¹ (III) 1s²2s²2p 3s²3p¹ Is²2s²2p 3s²3p³ a. I & II b. I, II & III 45. Which of the following comparisons of atomic radii (size) is incorrect? a) Na < Al b) Cs > Ba 46. Which of the following comparisons of ionization energy is incorrect? a) Na> K b) Ca> K c) Cs > K d) Mg < Al (II) (IV) c. I & III d. III & IV e. none of a to d c) N Ca
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 95AP
Related questions
Question
Plz give all answer with only little bit explanation
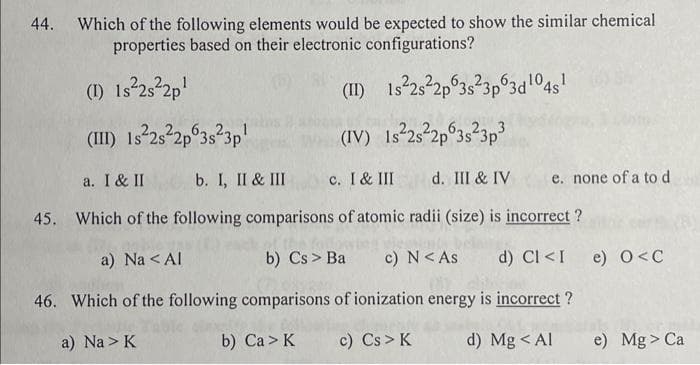
Transcribed Image Text:44. Which of the following elements would be expected to show the similar chemical
properties based on their electronic configurations?
(1) 1s²2s²2p¹
1s²2s²2p 3s²3p 3d 1¹04s¹
(II)
(III) 1s²2s²2p 3s²3p¹
(IV) Is²2s²2p 3s²3p³
c. I & III
a. I & II b. I, II & III
45. Which of the following comparisons of atomic radii (size) is incorrect?
c) N < As
a) Na < Al
b) Cs > Ba
46. Which of the following comparisons of ionization energy is incorrect?
a) Na> K
b) Ca> K
c) Cs > K
d) Mg < Al
d. III & IV e. none of a to d
d) CI <I e) O<C
e) Mg > Ca
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning