1. A chemist titrates 20.0 mL of 0.20 M HBrO (see Appendix) with 0.10 M NaOH. What is the pH: a) before any base id added? b) when [HBrO] = [BrO-]? c) at equivalence point?
1. A chemist titrates 20.0 mL of 0.20 M HBrO (see Appendix) with 0.10 M NaOH. What is the pH: a) before any base id added? b) when [HBrO] = [BrO-]? c) at equivalence point?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 110IL
Related questions
Question
1. A chemist titrates 20.0 mL of 0.20 M HBrO (see Appendix) with 0.10 M NaOH. What is the pH:
a) before any base id added?
b) when [HBrO] = [BrO-]?
c) at equivalence point?
d) when the amount (mol) of OH- added is twice the amount of HBrO present initially?
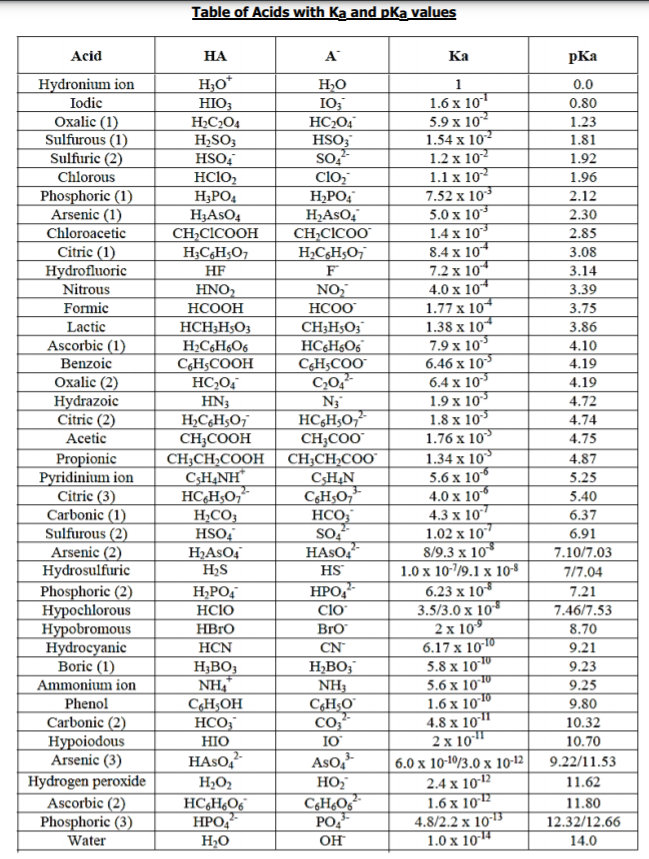
Transcribed Image Text:Table of Acids with Ka and pKa values
Acid
НА
A
Ка
pKa
H;O*
Hydronium ion
Iodic
Oxalic (1)
Sulfurous (1)
Sulfuric (2)
HO
IO;
HC2O4
HSO;
1
0.0
1.6 x 101
5.9 x 10
1.54 x 102
1.2 x 102
1.1 x 10
7.52 x 10*
5.0 x 10
1.4 x 10
8.4 x 10
7.2 x 10*
4.0 x 10
1.77 x 10*
1.38 x 10*
7.9 x 10
6.46 x 10
6.4 x 10
1.9 x 10
1.8 x 10°
1.76 x 10
1.34 x 10
5.6 x 10
4.0 x 10*
4.3 x 10"
1.02 x 10
8/9.3 x 10*
1.0 x 10-//9.1 x 10-8
6.23 x 10
3.5/3.0 x 10*
2х 10%
6.17 x 1010
5.8 x 1010
5.6 х 1010
1.6х 1010
4.8 x 10"
2 x 10"
| 6.0 x 10-1º/3.0 x 10-12
2.4 x 1012
1.6 x 10*12
4.8/2.2 x 1013
1.0 x 10
HIO3
0.80
H»C2O4
1.23
H;SO3
HSO;
HC1O,
H;PO4
H3ASO4
CH,CICOOH
H;C¿H;O7
HF
HNO,
НСООН
HCH3H§O3
H¿C¿HO6
C3HsCOOH
1.81
1.92
Chlorous
ClO,
H,PO,
1.96
Phosphoric (1)
Arsenic (1)
Chloroacetic
Citric (1)
Hydrofluoric
Nitrous
Formic
2.12
2.30
CH,CICOO
H,C¢H;O;¯
F
2.85
3.08
3.14
NO
3.39
HCOO
3.75
Lactic
CH;HsO;
3.86
Ascorbic (1)
Benzoic
Охalic (2)
Hydrazoic
Citric (2)
4.10
C¢H;COO"
4.19
C,0,
4.19
HN3
H,C¿H5O7¯
CH;COOH
CH;CH,COOH
C;H¾NH
N3
4.72
HC¿H;O;²
4.74
Acetic
CH;COO"
CH;CH,COO
C;H¾N
4.75
Propionic
Pyridinium ion
Citric (3)
Carbonic (1)
Sulfurous (2)
Arsenic (2)
Hydrosulfuric
Phosphoric (2)
Нурochlorous
Нуpobromous
Hydrocyanic
Boric (1)
Ammonium ion
Phenol
Carbonic (2)
Нурoiodous
Arsenic (3)
4.87
5.25
3-
5.40
6.37
H,CO}
HSO;
H;AsOq¨
H2S
HCO;
SO
HASO
6.91
7.10/7.03
HS
04.רוך
H,PO4
HCIO
НРО
Clo
7.21
7.46/7.53
HBrO
BrO"
8.70
HCN
CN
9.21
9.23
H;BO3
NH,"
C¢H;OH
HCO;
H;BO;
NH3
9.25
9.80
10.32
HIO
IO
10.70
Aso,
HO;
3-
HASO,²
9.22/11.53
Hydrogen peroxide
Ascorbic (2)
Phosphoric (3)
11.62
HC,H&O6¯
НРО
H;O
11.80
PO,
12.32/12.66
Water
OH
14.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning