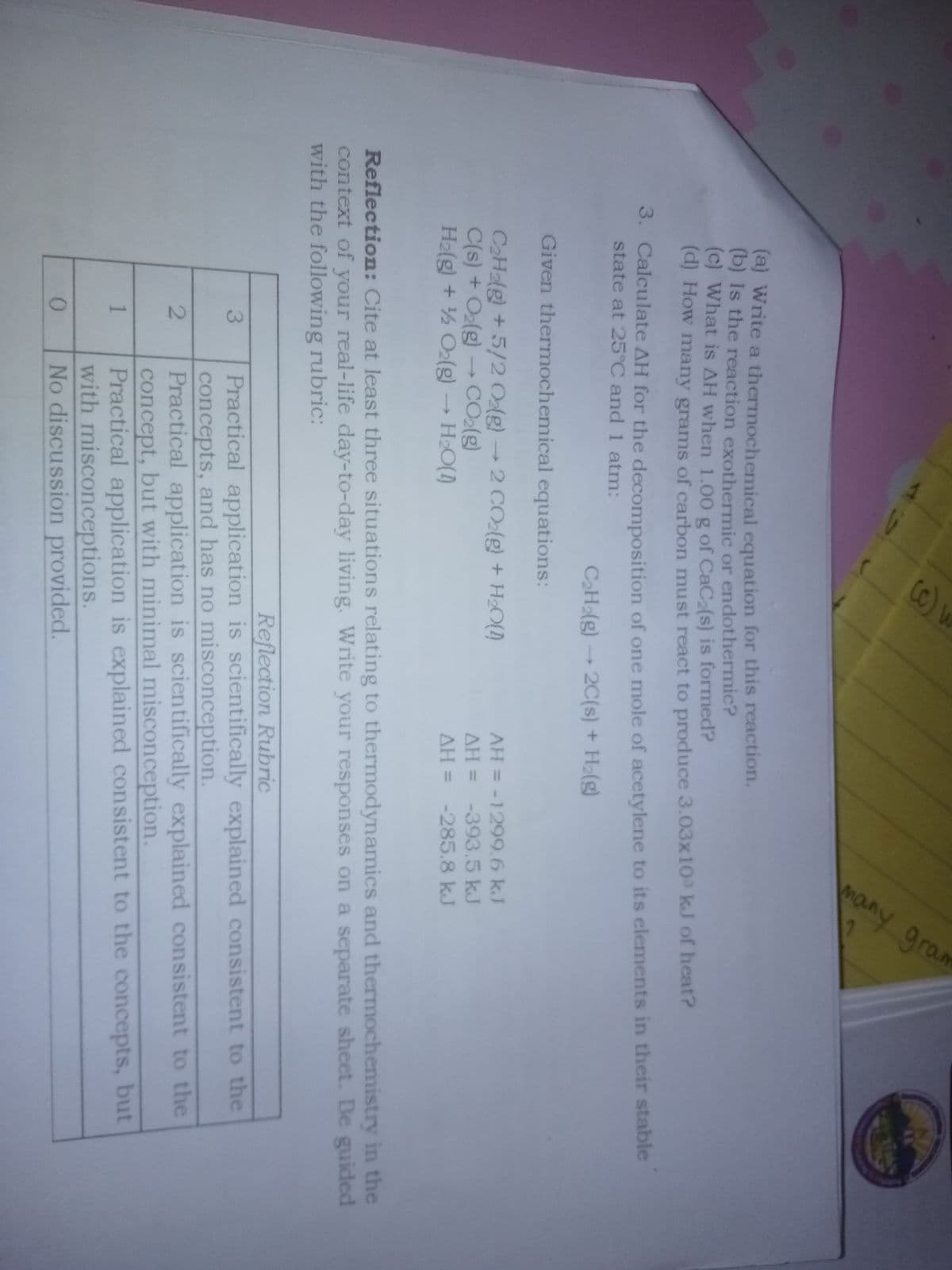
1. A gas is confined to a cylinder under constant atmospheric pressure. When 0.49 KJ of heat is added to the gas, it expands and goes 214 J of work on the surroundings. What are the values of ΔH and ΔE for this process? 2. Calcium carbide, CaC2, is the raw materials for the production of acetylene (used in wedding torches). Calcium carbide is produced by reacting calcium oxide with carbon. Carbon monoxide is also produced in this reaction. When one mole of calcium carbide is formed, 464.8 KJ is absorbed.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
1. A gas is confined to a cylinder under constant atmospheric pressure. When 0.49 KJ of heat is added to the gas, it expands and goes 214 J of work on the surroundings. What are the values of ΔH and ΔE for this process?
2. Calcium carbide, CaC2, is the raw materials for the production of acetylene (used in wedding torches). Calcium carbide is produced by reacting calcium oxide with carbon. Carbon monoxide is also produced in this reaction. When one mole of calcium carbide is formed, 464.8 KJ is absorbed.
(a) wite a thermochemical equation for this reaction.
(b) Is the reaction exothermic or endothermic?
(c) what is ΔH when 1.00 g of CaC2(s) is formed?
(d) How many grams of carbon must react to produced 3.03x10³ KJ of heat?
3. Calculate ΔH for the decompositiom of one mole of acetylene to its elements in their stable state at 25°C and 1 atm:
C2H2(g) = 2C(s) + H2(g)
Given thermochemical equation;
C2H2(g) +5/2 O2(g) = 2 CO2(g) + H2O(l)
C(s) +O2(g) = CO2(g)
H2(g) +½ O2(g) = H2O(l)
ΔH = -1299.6 kJ
ΔH = -393.5 Kj
ΔH = - 285.8 kj
4. Cite at least three situations relating tothermodynamics and thermochemistry in the context of your real life day-to-day living.
- 5. Some minerals are the nutrients that exist in tje body and some could be found naturally in the environment. As a student, how can you help in conserving and preserving the natural sources of these minerals like calcium and potassium?

Trending now
This is a popular solution!
Step by step
Solved in 3 steps









