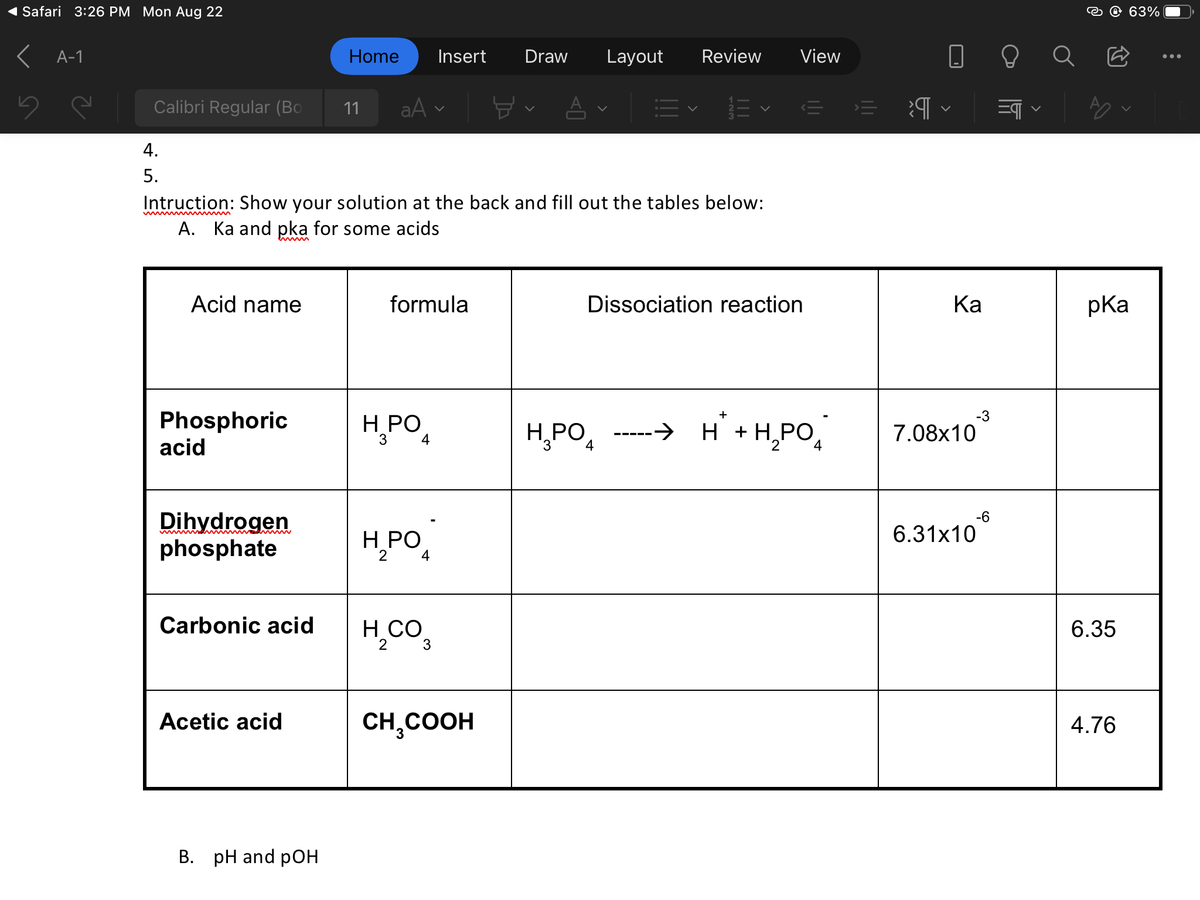
A. Ka and pka for some acids Acid name Phosphoric acid Dihydrogen phosphate Carbonic acid Acetic acid formula H₂PO4 3 H₂PO H, CO, 2 3 Dack and CH₂COOH out Dissociation reaction H₂PO4 →→ H² + H₂PO н Ka 7.08x10 -3 6.31x106 pka 6.35 4.76
A. Ka and pka for some acids Acid name Phosphoric acid Dihydrogen phosphate Carbonic acid Acetic acid formula H₂PO4 3 H₂PO H, CO, 2 3 Dack and CH₂COOH out Dissociation reaction H₂PO4 →→ H² + H₂PO н Ka 7.08x10 -3 6.31x106 pka 6.35 4.76
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
Show the solution
Transcribed Image Text:Safari 3:26 PM Mon Aug 22
< A-1
Calibri Regular (Bo
4.
5.
Acid name
Phosphoric
acid
Dihydrogen
phosphate
Carbonic acid
Intruction: Show your solution at the back and fill out the tables below:
A. Ka and pka for some acids
Acetic acid
Home Insert
B. pH and pOH
11
aAv
formula
H₂PO4
3
H₂PO4
2
H₂CO
2
3
B
CH₂COOH
Draw Layout Review View
H₂PO
Dissociation reaction
4
+
H² + H₂PO4
||
>
Ka
-3
7.08x10
6.31x10
-6
=¶
pka
6.35
63%
4.76
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

