1. (a) K₂Cr2O7 reacts with Fe(II) to produce Cr(III) and Fe(III) in acid medium. Write down the balanced equation and construct the cell. Calculate the standard potential and equilibrium constant for this reaction at 298 K? (Given standard reduction potentials, Eº for chromium system is 1.33 V and Eº for iron system is 0.77 V). (b) Also discuss the effect of pH on the change in potential for chromium system in acid medium.
1. (a) K₂Cr2O7 reacts with Fe(II) to produce Cr(III) and Fe(III) in acid medium. Write down the balanced equation and construct the cell. Calculate the standard potential and equilibrium constant for this reaction at 298 K? (Given standard reduction potentials, Eº for chromium system is 1.33 V and Eº for iron system is 0.77 V). (b) Also discuss the effect of pH on the change in potential for chromium system in acid medium.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 35P
Related questions
Question
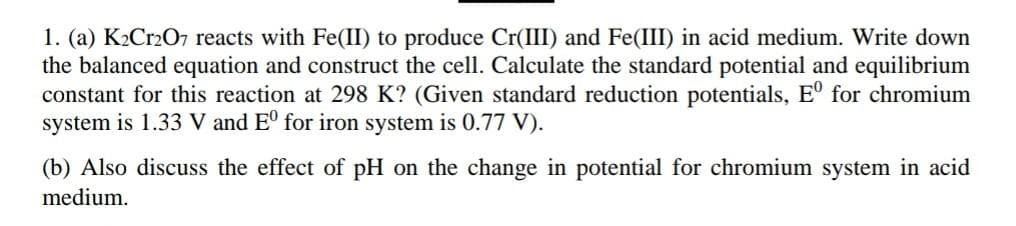
Transcribed Image Text:1. (a) K₂Cr2O7 reacts with Fe(II) to produce Cr(III) and Fe(III) in acid medium. Write down
the balanced equation and construct the cell. Calculate the standard potential and equilibrium
constant for this reaction at 298 K? (Given standard reduction potentials, Eº for chromium
system is 1.33 V and Eº for iron system is 0.77 V).
(b) Also discuss the effect of pH on the change in potential for chromium system in acid
medium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning